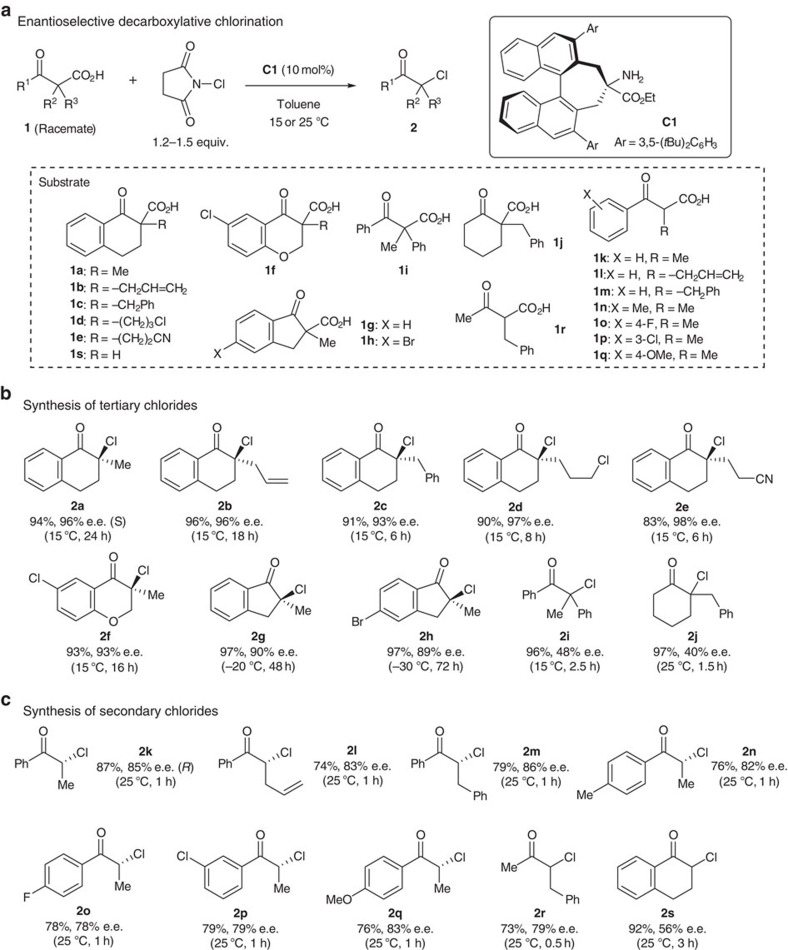
Figure 3. Scope of the catalytic enantioselective decarboxylative chlorination of β-ketocarboxylic acids.
(a) β-Ketocarboxylic acids were treated with NCS (1.2–1.5 equiv.) in the presence of catalyst C1 (10 mol%) and the mixture was stirred according to the conditions indicated in the figure in the dark. In all cases, the yield of 2 corresponds to the isolated yield of the purified compound, and the e.e. for each compound was determined by chiral high-performance liquid chromatography. (b) Enantioselective decarboxylative chlorination of α,α-dialkyl-β-ketocarboxylic acids (Me=methyl group, Ph=phenyl group). (c) Enantioselective decarboxylative chlorination of α-monoalkyl-β-ketocarboxylic acids. During the reaction, a solution of NCS in toluene was added slowly to a stirred solution of C1 and 1 in toluene over 1 h (0.5 h for the synthesis of 2r) using a syringe pump.