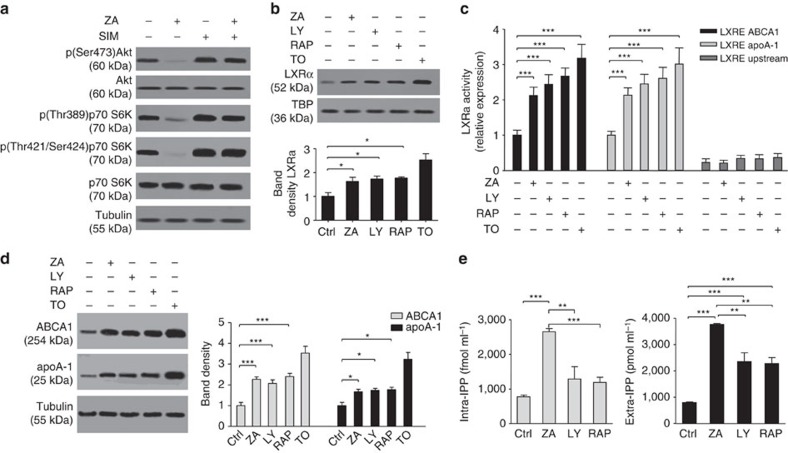
Figure 7. The PI3K/AkT/mTOR pathway regulates IPP release via LXRα/Abca1 activation.
(a) Targeting the Mev pathway fine-tunes the PI3K/Akt/mTOR signalling pathway. DC were left untreated or incubated with 1 μM ZA or simvastatin (SIM). ZA decreased, whereas simvastatin increased the signalling activity of the PI3K/Akt/mTOR pathway. (b) DC were grown for 24 h in the absence (−) or presence (+) of ZA (1 μM), the PI3K inhibitor LY294002 (LY, 200 μM for 24 h ), the mTOR inhibitor rapamycin (RAP, 20 nM for 24 h). The LXRα activator TO-901317 (TO, 100 nM for 24 h) was included as positive control. PI3K/mTOR inhibitors increased LXRα nuclear translocation, such as ZA. Pooled data are obtained by densitometric analysis with ImageJ software (http://imagej.nih.gov/ij/). The results are expressed as arbitrary units (n=3) (*P<0.05; ANOVA). (c) Evaluation of LXRα binding to LRE sequences in the Abca1 and apoA-1 promoters by ChIP assay. ZA, LY294002 and rapamycin increased Abca1 and apoA-1 promoters activity. The bars represent the mean±s.e.m. of three experiments (***P<0.001; ANOVA). (d) Western blot analysis of ABCA1 and apoA-1 expression in experimental conditions, as shown in b. PI3K and mTOR inhibition mimicked ZA treatment and upregulated ABCA1 and apoA-1 expression. β-tubulin and TBP were employed as a control of equal protein loading, as indicated. All blots are representative of out the of three experiments. Pooled data are obtained by densitometric analysis with ImageJ software (http://imagej.nih.gov/ij/). The results are expressed as arbitrary units (n=3). (*P<0.05, ***P<0.001; ANOVA). (e) Intracellular and extracellular IPP levels in DC after incubation in experimental conditions, as shown in b. As expected, PI3K/Akt/mTOR inhibition only increased extracellular IPP. The bars represent the mean±s.e.m. of three experiments (**P<0.01, ***P<0.001; ANOVA). ANOVA, analysis of variance.