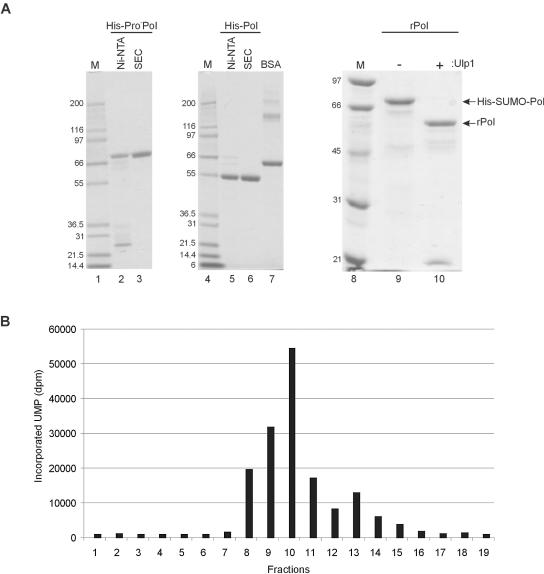
FIG. 2.
Purification of recombinant MD145-12 proteins. (A) SDS-PAGE analysis of His-Pro−Pol (lanes 2 and 3), His-Pol (lanes 5 and 6), His-SUMO-Pol (lane 9), and rPol (lane 10). Purification steps (Ni-NTA binding and SEC) and Ulp1 proteinase treatment are indicated above each lane. One microgram of His-Pro−Pol and His-Pol was separated by SDS-PAGE in a 4 to 12% Bis-Tris polyacrylamide gel (Invitrogen). One microgram of bovine serum albumin (BSA; Pierce) was added as control for the protein estimation (lane 7). Two and one-half micrograms of SUMO-Pol and rPol was resolved by SDS-PAGE in a 10% polyacrylamide gel. Proteins were visualized by staining with Coomassie blue. Lanes 1, 4, and 8 contain protein molecular weight markers (weights are at left in thousands). (B) RdRp assay of His-Pro−Pol fractions obtained during SEC. One microliter of each fraction was assayed in a 15-μl reaction mixture that contained 3 mM MgCl2, 10 μM UTP, and 5 μCi of [α-32P]UTP (400 Ci/mmol) for 20 min at 30°C. For this experiment and all subsequent experiments 200 μg of rifampin/ml was added. Other components of the RdRp assay mixture are described in Materials and Methods. Fraction numbers are indicated below the graph. UMP incorporation is given in disintegrations per minute (dpm) and corresponds for each fraction to an aliquot of 5 μl spotted on a DE81 filter.