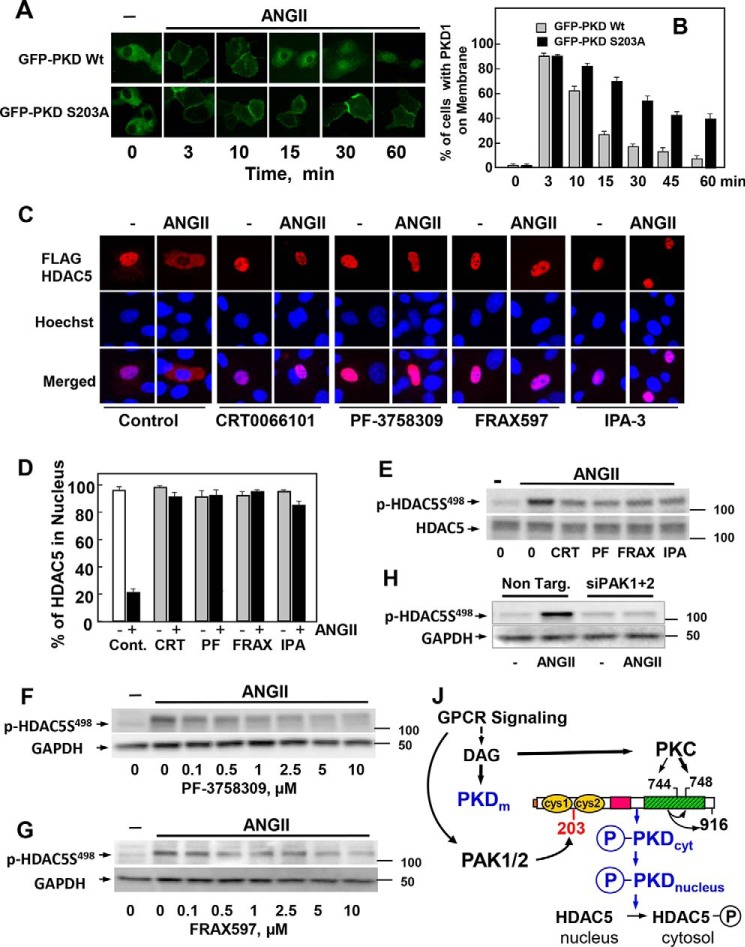
Figure 7.
Identification of a novel PAK/PKD/HDAC-signaling pathway. A, IEC-18 cells were transiently transfected with constructs encoding GFP-PKD1 or GFP-PKD1S203A. After 48 h the cultures were incubated in the absence (−) or presence of 10 nm ANG II for various times, as indicated. B, analysis of 70 cells at each time point. Results are expressed as the % of cells with GFP-PKD1 localized on the cell membrane. C, IEC-18 cells were transiently transfected with a plasmid encoding FLAG-tagged HDAC5. The cultures were incubated in the absence (−) or presence of 3.5 μm CRT0066101, 3.5 μm PF-3758309, 3.5 μm FRAX597, or 30 μm IPA-3 for 1 h before stimulation with 10 nm ANG II for 1 h. The cultures were then washed and fixed with 4% paraformaldehyde and stained with an antibody that detects the FLAG tag and Hoechst 33342 stain to visualize the nuclei. D, analysis of 100 cells for each treatment. Results are expressed as the % of cells with FLAG-HDAC5 localized in the nucleus. In each case the closed bars represent cultures stimulated with 10 nm ANG II. E, confluent cultures of IEC-18 cells were incubated in the absence (0) or in the presence of 3.5 μm CRT0066101 (CRT), 3.5 μm PF-3758309 (PF), 3.5 μm FRAX597 (FRAX), or 30 μm IPA-3 (IPA) for 1 h and then stimulated without (−) or with 10 nm ANG II for 15 min. F and G, IEC-18 cells were incubated in the absence (0) or in the presence of increasing concentrations of PF-3758309 (F) or FRAX597 (G) for 1 h and then stimulated without (−) or with 10 nm ANG II for 15 min. H, cultures of IEC-18 cells were transfected with non-targeting siRNA (Non. Targ) or with a mixture of siRNAs targeting PAK1 and PAK2 (siPAK1+2) for 5 days. Then the cultures were stimulated with 10 nm ANG II for 10 min. In E, F, G, and H, all incubations were terminated by the addition of 2× SDS-PAGE sample buffer, and cell lysates were resolved by SDS-PAGE. HDAC5 phosphorylation was determined by Western blot analysis using the antibody that detects its phosphorylated state on Ser498 and GAPDH as a loading control. J, GPCR signaling induces DAG accumulation at the plasma membrane, which mediates the translocation of inactive PKD1 from the cytosol to that cellular compartment. DAG also recruits and activates novel PKCs, which mediate the transphosphorylation of PKD1 on Ser744 (in mouse PKD1). DAG and PKC-mediated transphosphorylation of PKD1 act synergistically to promote PKD1 catalytic activation and autophosphorylation on Ser748. PAK1 and PAK2 also activated in response to GPCR signaling phosphorylate PKD1 at Ser203, thereby facilitating its dissociation from the membrane to the cytosol (PKDcyt) and to the nucleus (PKDnucleus), where PKD1 phosphorylates class IIa HDAC, including HDAC5 and HDAC7. In this manner the PAKs regulate class IIa HDAC phosphorylation and localization through phosphorylation of PKD1 on Ser203. Thus, our results identify a novel PAK/PKD/HDAC5 pathway in signal transduction. For more details, see “Discussion.”