Figure 7.
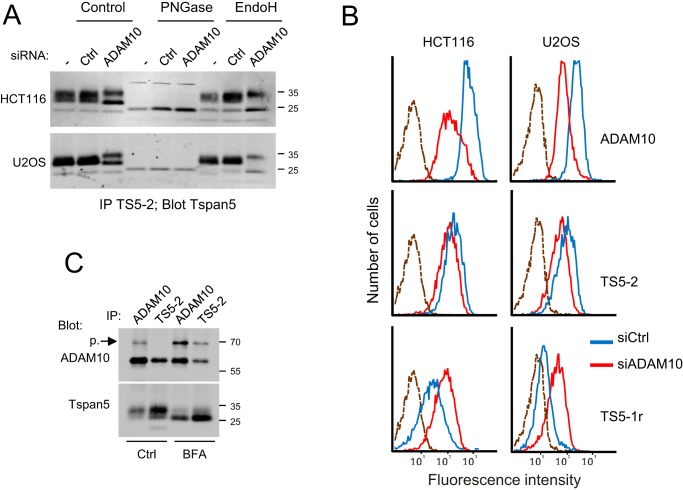
ADAM10 facilitates Tspan5 exit from the ER. A, HCT116 and U2OS cells were transfected or not with a control siRNA or an siRNA targeting ADAM10 and grown for 3 days before lysis in Brij 97, and immunoprecipitations with mAb TS5-2 were performed. The immunoprecipitated proteins were treated or not with PNGase F or EndoH, as indicated before electrophoresis and immunoblotting using biotin-labeled TS5-2 mAb. Note that the lower molecular weight form of Tspan5 reinforced after ADAM10 silencing is EndoH-sensitive. B, binding of mAb TS5-2 and TS5-1r to HCT116 and U2OS cells, as well as that of the anti-ADAM10 mAb 11G2, was analyzed by flow cytometry 3 days after transfection with a control siRNA or an siRNA targeting ADAM10. Note the decrease of the binding of TS5-2 and the increase of the binding of TS5-1r. The dashed line corresponds to the labeling with only the secondary reagent. C, HCT116 cells treated or not with BFA were lysed using Brij 97 before immunoprecipitations with the anti-ADAM10 mAb 11G2 or the anti-Tspan5 mAb TS5-2 and TS5-1r. The presence of Tspan5 and ADAM10 in the immunoprecipitates was visualized by immunoblotting using biotin-labeled TS5-2 and 11G2 mAbs. Note that TS5-2 co-immunoprecipitates the proform of ADAM10 after BFA treatment and that ADAM10 co-immunoprecipitates the immature form of Tspan5. The experiments in this figure have been done twice.