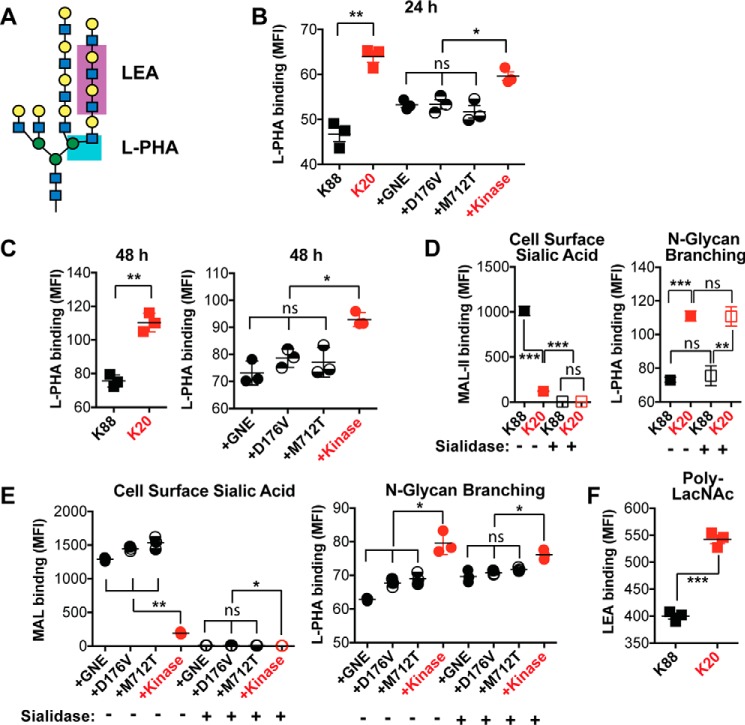
Figure 3.
Sialic acid production resulted in decreased N-linked glycan branching and decreased polyLacNAc extension. A, L-PHA lectin recognizes the β1–6-GlcNAc branch of tri- and tetra-antennary N-linked glycans, whereas LEA lectin recognizes polyLacNAc structures. Cell surface L-PHA lectin binding was measured by flow cytometry after culturing cells for 24 (B) or 48 (C) h. To test whether sialylation affects L-PHA binding, cells were cultured for 24 h, then treated with Arthrobacter ureafaciens sialidase to remove sialic acid. Desialylation was confirmed by measuring MAL-II lectin binding by flow cytometry; L-PHA lectin binding to desialylated cells was also measured by flow cytometry. K20 and K88 cells are compared in panel D, whereas K20 cells expressing wild-type and mutant forms of GNE are compared in panel E. Cells were cultured for 24 h, then cell-surface LEA lectin binding was measured by flow cytometry (F). Cell lines labeled in red did not express an active GNE epimerase domain and did not synthesize sialic acid. Data shown represent three biological replicates with error bars depicting the mean and S.E. Each data point represents the MFI of a single sample, typically of 10,000 cells. Statistical significance determined by unpaired Welch's test: *** indicates a p value < 0.001, ** indicates a p value < 0.01, and * indicates a p value < 0.05. ns indicates difference not statistically significant.