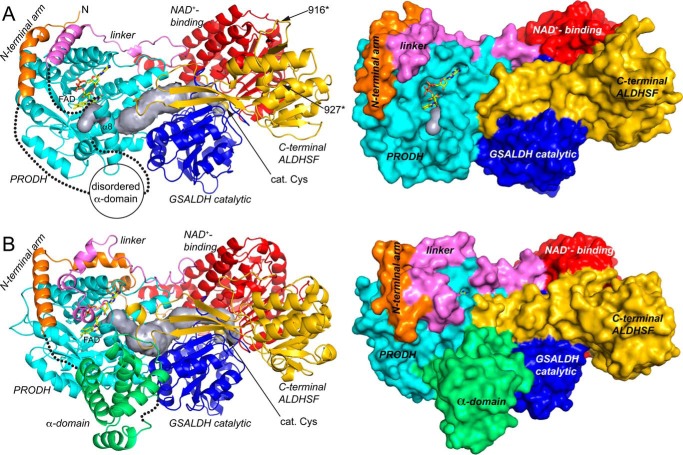
Figure 4.
Structure of the CfPutA monomer and comparison to SmPutA. A, backbone and surface representations of CfPutA. The domains have different colors, with the N-terminal arm in orange, PRODH barrel in cyan, PRODH-GSALDH linker in violet, GSALDH NAD+-binding in red, GSALDH catalytic in blue, and C-terminal ALDHSF in gold. The silver surface represents the substrate-channeling tunnel. The FAD is shown in yellow sticks. The dotted lines indicate disordered residues 33–118 (α-domain) and 421–434 (which connect α8 to the PRODH-GSALDH linker). The sites of domain deletion mutations are indicated as 916* and 927*. B, backbone and surface representations of SmPutA. The domains are colored as in A. The α-domain, which is disordered in CfPutA, is colored green.