Figure 3.
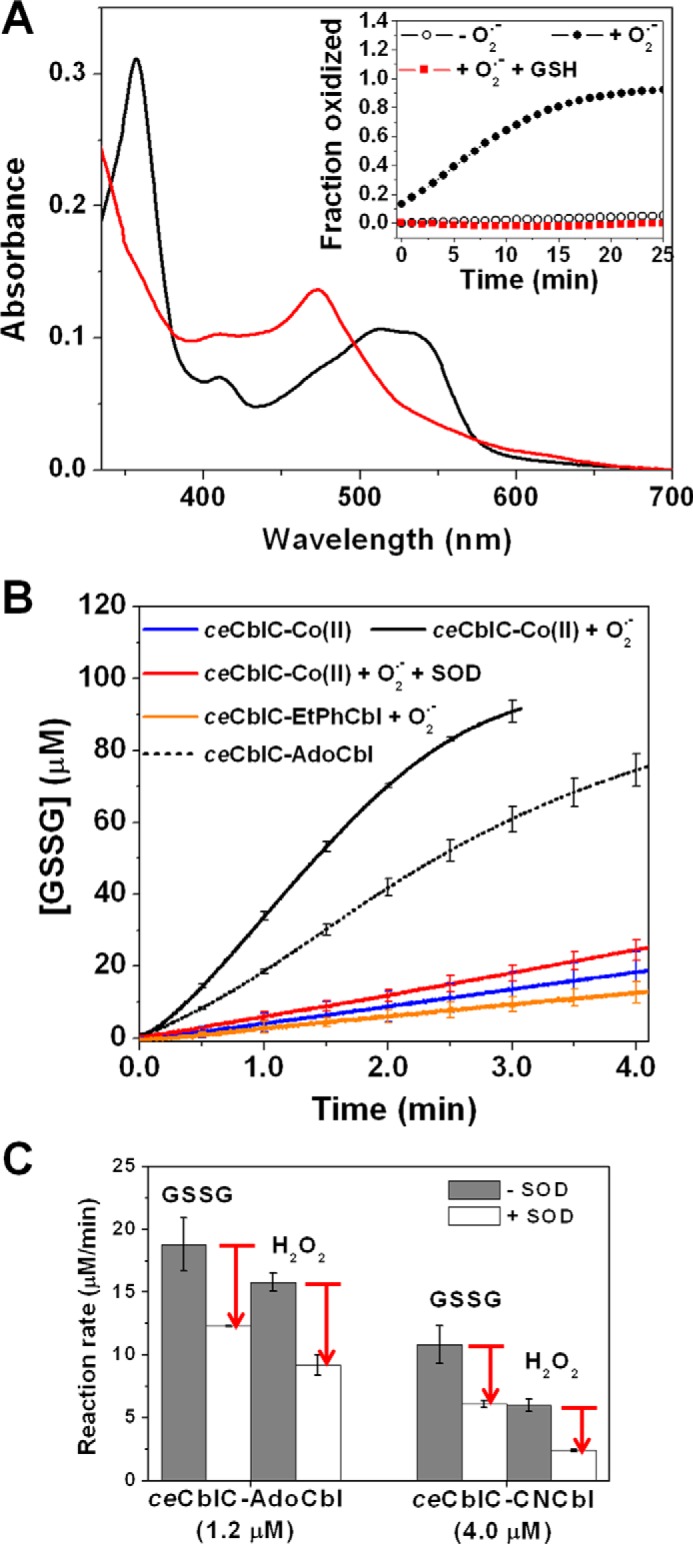
Superoxide is involved in futile redox cycling. A, absorption spectrum of ceCblC (40 μm)-bound cob(II)alamin (15 μm) after a 20-min incubation with O2˙̄ in the presence (red trace, λmax = 473 nm) and absence (black trace, λmax = 512 nm) of 4 mm GSH under aerobic conditions. Inset, time course of ceCblC-cob(II)alamin oxidation in the absence of O2˙̄ (open circles), presence of O2˙̄ (solid circles), or presence of 4 mm GSH and O2˙̄ (red squares). B, comparison of rates of GSSG production by ceCblC-cob(II)alamin, ceCblC-AdoCbl, and ceCblC-EtPhCbl, each with 1.2 μm cobalamin and 2.5 μm ceCblC, in the presence of 4 mm GSH. C, SOD (500 units/ml) partially inhibits redox cycling of ceCblC-AdoCbl and ceCblC-CNCbl in the presence of 4 mm GSH. The O2˙̄ flux was 1.2 μm/min in A and B. [SOD] = 500 units/ml in A–C. The data represent the mean ± S.D. (error bars) of three independent experiments.
