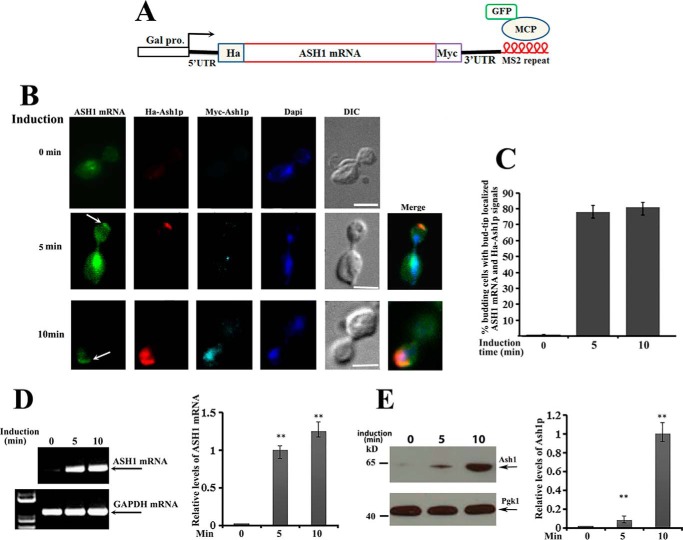
Figure 1.
Translation of ASH1 mRNA occurs after bud-tip localization. A, schematic presentation of the centromeric construct used to visualize ASH1 mRNA localization and translation in ash1 cells. The ASH1 gene was placed under the control of a galactose-inducible promoter (Gal1). HA and Myc tags were separately fused onto the N and C termini of Ash1. Six repeats of MS2 stem loops were fused to the 3′-UTR of ASH1 mRNA, which were recognized by GFP-MCP (MS2 RNA coat protein). B, yeast cells were cultured in 2% raffinose medium at 30 °C and induced with galactose (2%). After 0, 5, and 10 min of induction, cells were harvested and subjected to immunostaining assays. Localization of ASH1 mRNA was visualized by GFP-MCP, and translation of Ash1 was detected by Cy3-labeled HA (red) or Cy5-labeled Myc (cyan) antibodies. The arrows indicate the bud-tip-localized ASH1 mRNA. Bar, 3 μm. C, percentage of anaphase cells with localized ASH1 mRNA and translated HA-Ash1 signals after galactose induction from three independent experiments. An average of 50–60 cells were counted each time. D, RT-PCR was used to detect the expression of ASH1 mRNA after 0, 5, and 10 min of galactose induction. GAPDH mRNA was used as a positive control. Right, error bars indicate S.E. from three independent experiments. **, p < 0.01. E, full-length Ash1 after 0, 5, and 10 min of galactose induction was detected using anti-Myc antibodies by Western blotting, in which Pgk1 was used as a loading control. Right, mean values ± S.D. (error bars) were from three independent experiments. **, p < 0.01.