Figure 6.
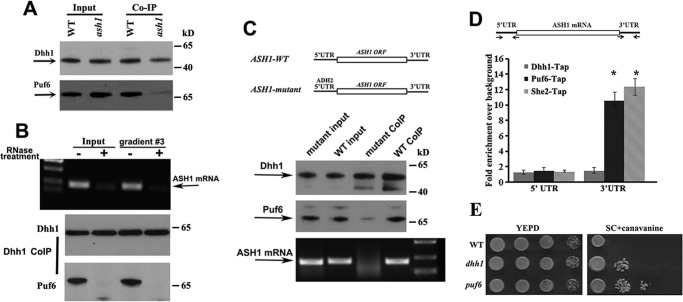
Dhh1 does not directly interact with Puf6; it could bind to the 5′-UTR of ASH1 mRNA. A, co-IP experiments using Protein A beads conjugated with Dhh1 antibodies were performed in the extracts of WT and ash1 cells. Western blotting indicated that co-precipitation of Dhh1 with Puf6 required ASH1 mRNA. B, co-IP experiments were performed in the yeast extracts and the non-polysomal fraction 3 treated with or without RNase A and RNase One. Dhh1 did not co-precipitate Puf6 when total mRNA in the extracts was degraded. C, top panels, schematic presentation of yeast strains expressing ASH1-WT (5′UTR-ASH1–3′UTR) and ASH1-mutant (ADH2–5′ UTR-ASH1-3′UTR) mRNAs. The 5′-UTR of ASH1 is a 150-nucleotide sequence upstream of the translational initiation site of the mRNA. The 3′-UTR of ASH1 is a 160-nucleotide sequence containing binding sites for Puf6. The 5′-UTR of ADH2 is a 150-nucleotide sequence upstream of the translational initiation site of the mRNA. Bottom panels, Dhh1 was immunoprecipitated in the extracts of ASH1-WT and ASH1-mutant cells. Puf6 and ASH1 mRNA were detected in the precipitates of WT extracts. A small amount of Puf6 and no ASH1 mRNA were detected in the precipitates of ASH1-mutant extracts. Arrows, positions of Dhh1, Puf6, and ASH1 mRNA. The results are representative of three independent experiments. D, top, schematic presentation of the ASH1 gene. The 3′-UTR contains the E3 localization element for Puf6 and She2 binding. Arrows, primers used for qPCR analysis. Amplicon lengths for 5′-UTR and 3′-UTR are 150 and 160 bp, respectively. Bottom, TAP-tagged Puf6, She2, and Dhh1 were used for CHIP assays. After immunoprecipitation, amplicons corresponding to 5′-UTR and 3′-UTR were amplified and analyzed. E, effect of DHH1 deletion on repression of the HO promoter. 10-Fold serial dilutions of exponentially growing wild-type, dhh1, or puf6 cells were spotted on YPD or SC medium containing 0.03% canavanine and incubated for 2 and 5 days at 30 °C, respectively. Error bars, S.E.