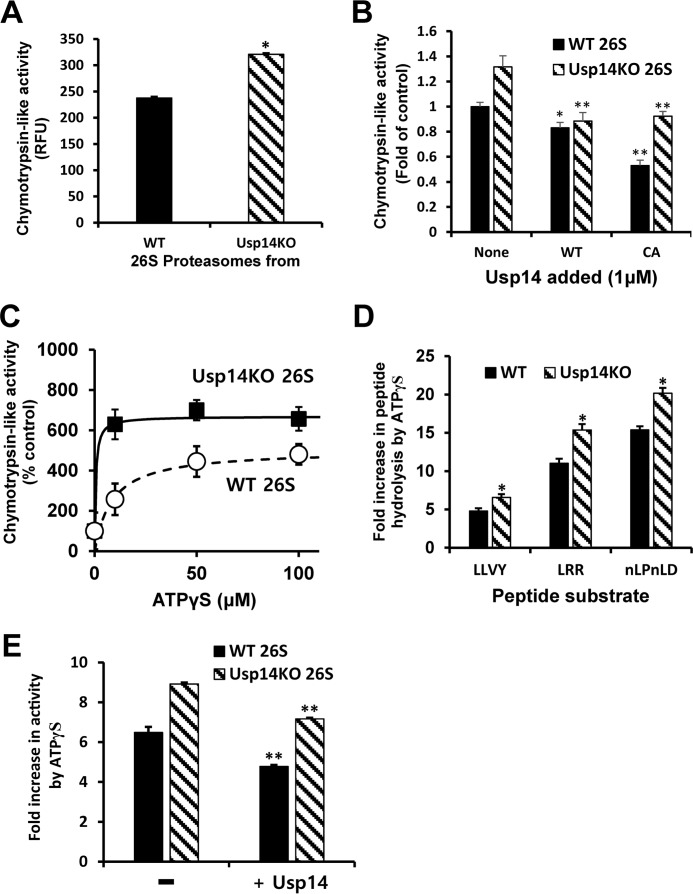
Figure 2.
Usp14 reduces peptidase activities in the proteasome and the activation of peptide entry by ATPγS. Peptidase activities of purified WT or Usp14KO 26S were assayed by measuring hydrolysis of fluorogenic peptide-amc substrates (10 μm; Suc-LLVY-amc for chymotrypsin-like activity, Boc-LRR-amc for trypsin-like activity, and Ac-nLPnLD-amc for caspase-like activity) in the presence of 100 μm ATP or ATPγS. The basal peptidase activity (control) of the proteasomes was measured in the presence of ATP. Error bars represent S.D. of at least three independent measurements. A, chymotrypsin-like activity of 26S purified from Usp14KO cells (4 nm) is consistently greater (35%) than that of WT 26S in the presence of ATP (100 μm) even though only a small fraction (∼40%) of the proteasomes from WT cells contained Usp14 (Fig. 1A). Cleavage of the fluorogenic substrate Suc-LLVY-amc (10 μm) by each type of proteasomes was monitored. *, p < 0.05 compared with WT 26S by Student's t test. B, binding of full-length recombinant Usp14 protein to the 26S particles inhibits the chymotrypsin-like activity of WT and Usp14KO 26S. Purified proteasomes (100 nm) were preincubated for 10 min at room temperature with bacterially expressed full-length WT or an inactive Cys → Ala (CA) mutant Usp14 (1 μm) before the start of the assays. *, p < 0.05; **, p < 0.01 compared with control proteasomes (without Usp14 added) by Student's t test. C, increasing amounts of ATPγS stimulate the chymotrypsin-like activity of both WT and Usp14KO proteasomes in a saturable manner. Both the maximal stimulation by ATPγS and the apparent affinity for ATPγS were greater for Usp14KO 26S than for the WT particles. D, ATPγS (100 μm) markedly stimulated all three peptidase activities of both WT and Usp14KO proteasomes (4 nm) but consistently caused a 30–40% larger stimulation of these activities in the Usp14KO 26S. *, p < 0.05 compared with WT 26S by Student's t test. E, addition of Usp14 reduced the stimulation of the chymotrypsin-like activity by ATPγS. After incubation of purified proteasomes (100 nm) with a molar excess of Usp14 (1 μm) at room temperature for 10 min, the chymotrypsin-like activities of WT and Usp14KO 26S with ATPγS (100 μm) were assayed as in D. **, p < 0.01 compared with control samples without Usp14 added by Student's t test. RFU, relative fluorescence units.