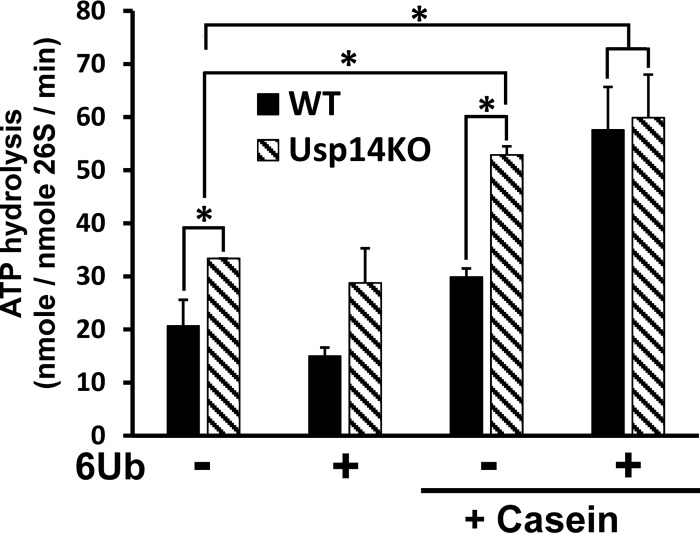
Figure 3.
ATPases of Usp14KO proteasomes, unlike those of WT 26S, are stimulated by unfolded proteins in the absence of a Ub chain. ATP hydrolysis by 26S proteasomes (20 nm) was measured using the malachite green assay (54). The basal ATPase activity of Usp14KO 26S was consistently (60%) higher than that of WT 26S. Upon addition of casein (1 μm) and a hexa-Ub chain (1 μm), which together mimic the binding of a Ub conjugate (22), ATP hydrolysis of WT 26S increased 2–3-fold and resembled the ATPase activity of Usp14KO 26S under the same conditions. However, hexa-Ub alone did not increase ATP hydrolysis of either type of proteasomes. ATP hydrolysis by Usp14KO 26S, but not that by WT 26S, was enhanced by addition of casein alone and reached the same level as seen with 26S upon binding both a Ub chain and casein. 6Ub, linear hexa-Ub chain. *, p < 0.05 compared with control by Student's t test. Error bars represent S.D.