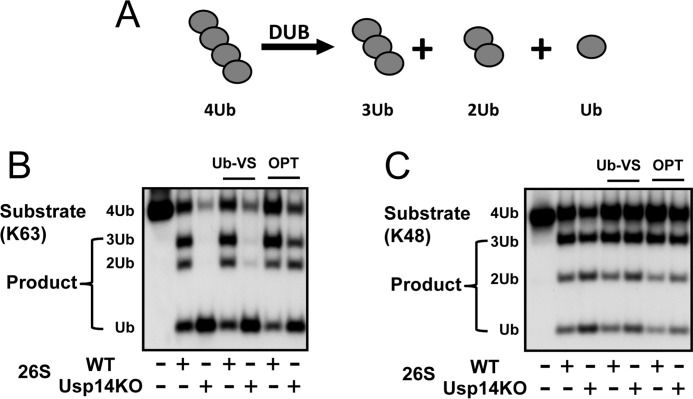
Figure 5.
Usp14 inhibits deubiquitination by Rpn11. A, disassembly of tetra-Ub chain (368 nm) by WT or Usp14KO 26S (5 nm) was assayed after incubation at 37 °C for 20 min. The generation of tri-, di-, and mono-Ubs was analyzed by Western blotting after electrophoresis. B and C, Usp14KO MEF 26S proteasomes disassembled tetra-Ub chains much faster than WT 26S particles. Although Ub-VS (1.5 μm) did not inhibit Ub chain hydrolysis, o-phenanthroline (OPT) (1 mm) markedly inhibits Ub chain hydrolysis and reduces deubiquitination by Usp14KO 26S to the level of WT 26S. Thus, this process involves the Zn2+ metalloprotease Rpn11. Similar results were also obtained with Lys-63 tetra-Ub chains (B) and Lys-48 tetra-Ub chains (C) as substrates and were obtained in at least three independent experiments.