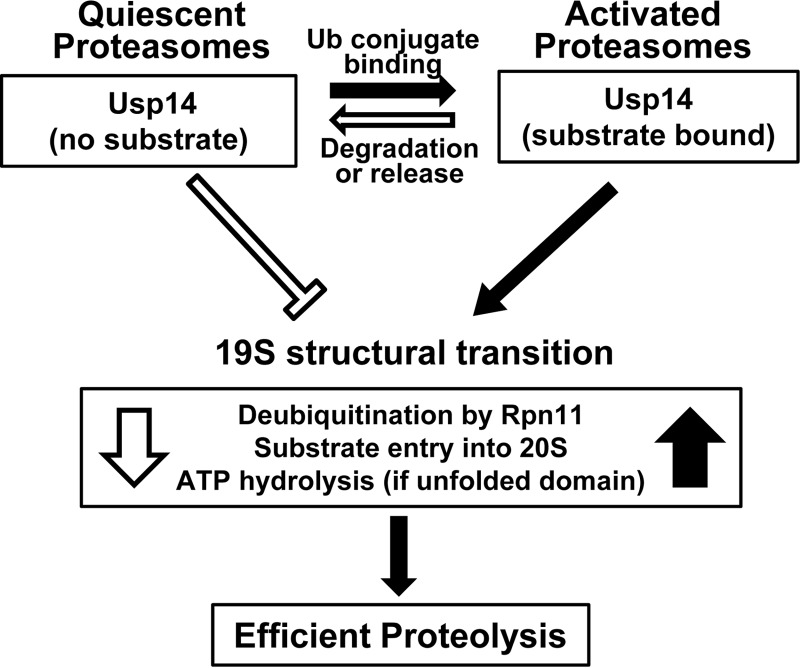
Figure 6.
Summary of Usp14's allosteric regulation of proteasomal degradation. Without a Ub conjugate bound, proteasomes are relatively inactive, and Usp14 inhibits ATP hydrolysis, substrate entry into the 20S, and deubiquitination by Rpn11. These actions reduce futile ATP consumption and the degradation of non-ubiquitinated proteins, which occur in the Usp14KO 26S but not in WT 26S. However, upon Ub chain binding, Usp14 (and the 19S particle) undergoes a major structural transition and thus activates Rpn11, peptide entry into 20S, and ATP hydrolysis (if an unfolded protein also is bound). These actions promote efficient degradation of ubiquitinated substrates. This activated state should be maintained as long as Usp14 binds a Ub chain. These processes return to the quiescent basal state when the substrate is degraded or is deubiquitinated and dissociates. These allosteric actions are not dependent on Usp14's catalytic activity, which functions to degrade the Ub chain and thus limits the duration of the active state and the substrate's “dwell time” on the 19S complex.