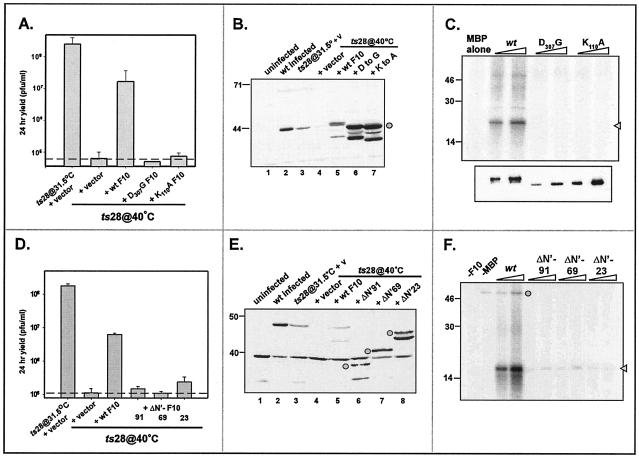
FIG. 8.
Transient complementation of nonpermissive ts28 infections. (A to C) The kinase activity of F10 is required for its biological function in vivo. BSC40 cells were infected with ts28 (MOI = 5) at 40°C and then transfected at 6 hpi with an empty vector or plasmids directing the expression of either wt F10 or kinase-null alleles of F10 (K110A F10 or D307G F10). As a positive control, an additional culture was infected at 31.5°C and transfected with an empty vector. At 28 hpi, the cells were harvested and viral yields were titrated at 31.5°C to evaluate the restoration of virus production (A). The data represent the average of two experiments, and the dashed line indicates the baseline titer obtained from cells transfected with the empty vector. Only the wt F10 construct restored virus production in the context of a nonpermissive ts28 infection. Extracts were also subjected to immunoblot analysis with the anti-F10 antiserum to verify the stable expression of the plasmid-borne proteins (B, lanes 5 to 7, circle). Endogenous F10 protein was also detected in wt-infected cells (lane 2) or cells infected with ts28 at the permissive temperature (lane 3). To verify that the K110A and D307G substitutions did in fact disrupt enzymatic activity, we tested equivalent amounts of purified recombinant N′His-wt F10, -K110A F10, and -D307G F10 proteins for their ability to phosphorylate MBP in vitro (C); an autoradiograph of the kinase assays (top) and an immunoblot developed with a His probe (bottom) are shown. No phosphorylation of MBP was seen in the reactions performed with N′His-K110A or -D307G F10. (D to F) The N terminus of F10 is required for its biological function in vivo and its enzymatic activity in vitro. Cells were infected as described above and then transfected with an empty vector or plasmids directing the expression of either wt F10 or alleles containing N-terminal deletions (ΔN′-F10) of 91, 69, and 23 aa. The 28-h viral yield was determined by titration at 31.5°C (D), and the stable expression of the various proteins was confirmed by immunoblot analysis with the anti-F10 serum (E) (the ΔN′-F10 proteins are indicated by circles). The ∼38-kDa protein seen in all samples in panel E represents a cross-reactive cellular protein. Only the wt F10 construct restored virus production in the context of a nonpermissive ts28 infection. To test the impact of the N-terminal deletions on enzymatic activity, we tested increasing amounts of purified 3XFLAG-tagged wt F10 and ΔN-F10 proteins (4 to 20 ng) for their ability to phosphorylate MBP in vitro (F). Phosphorylated MBP is indicated by the arrowhead; autophosphorylated 3XFLAG-F10 is indicated by the circle. The ΔN-F10 proteins showed significant reductions in specific activity compared to wt F10. For panels B, C, E, and F, the electrophoretic migration of protein standards is shown at the left, with molecular masses indicated in kilodaltons.