Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by development of autoantibodies to nuclear and cytoplasmic antigens. A small subset of SLE patients who had the typical clinical features of SLE was reported to show persistently negative antinuclear antibody tests. Our report describes a 5-year-old male who presented with histopathological findings suggestive of lupus nephritis with no clinical signs or symptoms of SLE and negative autoantibodies. He was treated with corticosteroids, mycophenolate mofetil, and monthly intravenous cyclophosphamide. During the 2-year follow-up period, the proteinuria resolved and kidney function improved with continued negative autoantibody workup. This case presents a category of renal-limited “lupus-like” glomerulonephritis which can be challenging to treat and carries a poor prognosis.
Keywords: Antinuclear antibodies, Lupus-like nephritis, Full-house
Introduction
Lupus nephritis (LN) is a frequent and serious complication in lupus patients. It carries poor prognosis and increased risk of end-stage kidney disease, especially in children. Renal-limited “lupus-like” nephritis is characterized by histopathological findings consistent with LN but without clinical or serological evidence of systemic lupus erythematosus (SLE). The pathogenesis, classification, clinical course, and treatment of renal-limited “lupus-like” nephritis are still unclear. In this article, we present a child with renal-limited “lupus-like” nephritis with no extrarenal manifestations or serological evidence of SLE. We review the literature and highlight some of the possible mechanisms that may explain this condition.
Case Presentation
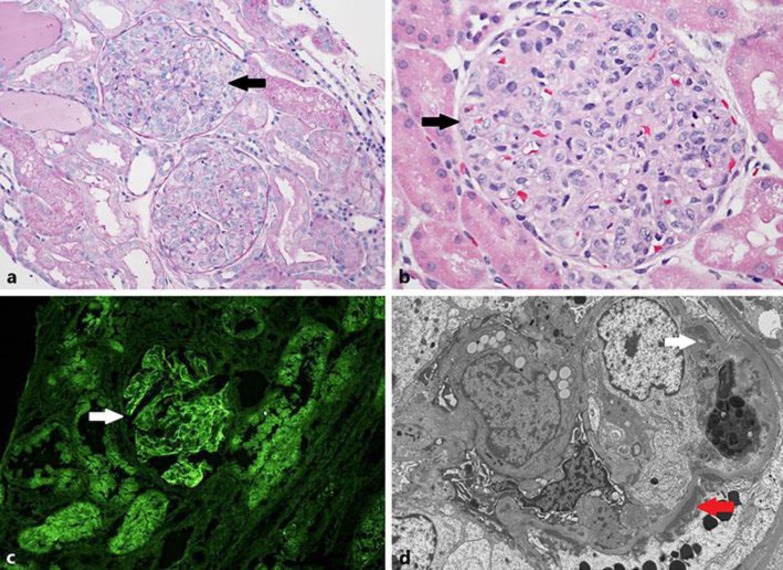
A 5-year-old Hispanic male with no family history of renal or autoimmune disease presented with an acute onset of respiratory symptoms with 1 day of fever, sore throat, and cough. The patient rapidly developed edema, proteinuria, low urine output, elevated creatinine (1.5 mg/dL), and new-onset hypertension (above 95 percentile for age, gender, and height). Evaluation for streptococcal pharyngitis was negative. C3 and C4 complement levels were low, while antinuclear antibody (ANA), anti-double stranded DNA (anti-dsDNA), anti-Ro/SSA, anti-La/SSB, anti-Sm, anti-U1 RNP antibodies, and ANCA serologies were negative. Physical examination was only significant for facial edema. A renal biopsy was performed, and light microscopy showed diffuse, global endocapillary hypercellularity (Fig. 1a, b). Direct immunofluorescence demonstrated “full-house” staining with diffuse, global, granular staining of glomerular capillary walls by IgG (4+ on a scale of 0–4, Fig. 1c), IgA (3+), IgM (2+), C1q (2+), and C3 (4+). Electron microscopy showed abundant subendothelial immune complex-type electron-dense deposits (Fig. 1d). Smaller numbers of subepithelial and mesangial deposits were also seen, but tubuloreticular inclusions (TRIs) were not identified. The patient's creatinine and hypertension improved with supportive measures and diuretics. One month later, he was readmitted after developing gross hematuria, nephrotic-range proteinuria, and rapidly progressive renal failure with creatinine of 3.8 mg/dL, requiring hemodialysis. He continued to have negative autoantibodies and ANCA serologies with normal C3 and C4 levels. There was no evidence of HIV, HBV, or HCV infection and no signs or symptoms of SLE. The decision was made to treat him as a “lupus nephritis” patient based on his kidney biopsy, and he was treated with a 3-day course of methylprednisolone pulse therapy along with IV cyclophosphamide. He completed 6 doses of monthly IV cyclophosphamide and was transitioned to mycophenolate 300 mg/m2/dose twice daily with continued oral steroids. Proteinuria and gross hematuria resolved, and creatinine has been normal (0.4 mg/dL) over the period of 2-year follow-up with continued negative lupus serology and no signs or symptoms of SLE on periodic screening.
Fig. 1.
Renal biopsy findings. a Glomeruli with diffuse, global endocapillary hypercellularity (periodic acid-Schiff stain, ×200). b Glomeruli with diffuse, global endocapillary hypercellularity (hematoxylin and eosin stain, ×400). c Immunofluorescence microscopy shows positive IgG staining within a glomerulus (×40). d Electron microscopy shows abundant subendothelial immune complex-type electron-dense deposits (white arrow). Smaller numbers of subepithelial (red arrow) and mesangial deposits were also seen. Tubuloreticular inclusions were not identified.
Discussion
SLE is a chronic autoimmune inflammatory disease characterized by loss of tolerance against self-antigens, polyclonal autoantibody production, immune complex formation, and activation of complement leading to inflammation and multiorgan injury. The etiology of SLE has been related to genetic, epigenetic, environmental, hormonal, and immune factors. Loss of self-tolerance to nuclear autoantigens becomes evident by the presence of ANAs [1]. Apoptosis, necroptosis, and autophagy have also been implicated to play roles in immune dysregulation and organ damage.
The histopathological features of LN have been classified by the World Health Organization and the International Society of Nephrology/Renal Pathology Society (ISN/RPS) [2]. The finding of a “full-house” pattern of staining (IgG, IgA, IgM, C1q, and C3) by immunofluorescence supports a diagnosis of LN. Electron microscopy demonstrates a combination of mesangial, subendothelial, and/or subepithelial electron-dense deposits which vary according to class. TRIs are typically present within the cytoplasm of endothelial cells.
Elevated ANA and anti-dsDNA antibody titers, and decreased serum complements are the hallmark laboratory tests of lupus yielding a combined sensitivity for the diagnosis of SLE exceeding 90% [2, 3]. Autoantibodies are typically present many years before the diagnosis of SLE, and the appearance of autoantibodies in patients with SLE tends to follow a predictable course, with a progressive accumulation of specific autoantibodies before the onset of SLE, while patients are still asymptomatic [3, 4].
Arbuckle et al. [4] described 3 distinct phases of autoantibodies during lupus development. In the first phase, patients are asymptomatic and they do not have any detectable autoantibody levels (e.g., ANAs, anti-Ro, anti-La, and antiphospholipid antibodies). The second phase occurs when patients develop detectable autoantibodies without clinical manifestations. In the final phase, patients have detectable autoantibodies with development of signs and symptoms of SLE.
Autoantibodies play a key role in development of LN by reacting with antigens to form immune complexes in the kidney. The exact mechanisms of formation of these immune complexes are not well understood. The “planted antigen theory” states the formation of the complexes is related to deposition of circulating autoantigens within the glomeruli. The “cross reactive theory” contends the complexes result from binding of autoantibodies to in-situ glomerular antigens such as laminin, annexin II or heparan, and/or anti-dsDNA/chromatin antibodies binding to nucleosomes/DNA present in the glomerular matrix. These immune complexes can cause activation of the complement cascade and/or co-stimulation of FcγRs and endosomal Toll-like receptors resulting in generation of local cytokines, chemokines, and adhesion molecules. This leads to further influx of inflammatory cells and production of proinflammatory cytokines causing renal inflammation, tissue injury, and fibrosis [5].
Although detection of serum autoantibodies is considered a hallmark for clinical diagnosis of SLE, it is shown that autoantibodies to classic lupus antigens are neither required nor sufficient for end-organ damage. Patients with SLE and negative autoantibodies have been reported, indicating that the lack of positive serologies does not exclude SLE [6,7,8].
Other possible explanations for negative serology in full house “lupus-like” nephritis can be related to laboratory techniques. Levels of ANA and/or autoantibodies too low to detect by conventional laboratory assays may be a cause. A longer follow-up period may be needed to detect lupus antibodies in some patients. Development of autoantibodies other than the frequently tested ones, or ANAs becoming entrapped in circulating immune complexes, are additional possibilities.
The clinical course of full house “lupus-like” nephritis is still unclear. There are multiple reports of patients who initially presented with a “lupus-like” glomerulonephritis (GN), and after a variable period of time some developed features of SLE clinically and serologically. Others continued with no further signs or symptoms of lupus and negative serologies.
A report by Jones and Magil [9] described 4 females and 1 male, between the ages of 19 and 38 years who developed renal-limited glomerular disease. All presented with proteinuria, and biopsies revealed mesangial and focal proliferative GN with “full-house” immunofluorescence. Electron microscopy revealed the deposits were located primarily in the mesangium, and TRIs were not identified. After 10–58 months (mean 2.3 years) of follow-up, none demonstrated any evidence of systemic illness.
Gianviti et al. [10] reviewed 31 children with “full-house” nephropathy. Fourteen of the children were diagnosed with SLE by autoantibody positivity and other clinical criteria for lupus. Three patients had negative serologies for lupus, but developed autoantibodies during the follow-up period. Fourteen children remained seronegative and did not develop clinical signs and symptoms of SLE over a mean follow-up time of 5.8 years (median follow-up 4.9 years). All of the patients received early immunosuppressive therapy consisting of corticosteroids with additional immunosuppressive agents (e.g., cyclophosphamide, chlorambucil, cyclosporine, or azathioprine). More than 50% of patients had renal dysfunction at the end of follow-up, including 5 patients with chronic kidney disease and persistent proteinuria, and 1 patient who progressed to end-stage renal disease.
Baskin et al. [11] reported a 10-year-old female with declining renal function and renal biopsy showing “full-house” nephropathy with negative serologies (complement levels, autoantibodies, and ANCA) that also lacked clinical signs and symptoms of SLE. Wen and Chen reviewed 24 patients with “full-house” nephropathy in the absence of clinical or serological evidence of SLE at the time of renal biopsy. The histopathological diagnoses included membranous GN (46%), IgA nephropathy (21%), membranoproliferative GN (12.5%), postinfectious GN (12.5%), C1q nephropathy (4%), and unclassified mesangial GN (4%). TRIs were not observed in any patient. One of the patients with membranous GN and all of the IgA nephropathy patients subsequently developed lupus [12].
Huerta et al. [13] reported 4 female adult patients with renal biopsy that showed IgG-dominant immune-complex-mediated GN with variable co-deposits of IgA, IgM, C3, and C1q highly suggestive of LN, but without extrarenal manifestations or serologies of SLE at the time of biopsy or over 3 years of follow-up. Extensive workup showed negative serologies for HBV, HCV, and HIV with no apparent etiology. All patients in this study received steroids, and 3 patients also received mycophenolate (MMF) and/or cyclophosphamide. Despite aggressive therapy, 3 of the 4 patients had a progressive decline in renal function leading to ESRD.
Caltik et al. [14] reported a 13-year-old boy who presented with pretibial edema, arthritis, and petechia on bilateral ankles. The patient had elevated creatinine (1.65 mg/dL), hypocomplementemia, nephrotic range proteinuria, hematuria, and pleural effusion. ANA, autoantibodies, and ANCA serologies were negative. A renal biopsy revealed diffuse proliferative GN with a “full-house” staining pattern by immunofluorescence microscopy examination suggesting class IV LN. He was treated with a total of 6 courses of monthly intravenous pulse methylprednisolone, dipyridamole, and oral cyclophosphamide followed by azathioprine and oral prednisolone therapy. The patient improved and follow-up did not show any progression to SLE.
A recent retrospective observational study reported the outcome of Italian children and adolescents with renal-limited “full-house” LN. The study showed a significant proportion of these patients who presented with typical histological features of LN and consequently treated as LN, had a good renal outcome. The authors found that younger age, female gender, and development of systemic symptoms suggestive of SLE may increase the risk of progressive chronic kidney disease and eventually ESRD [15].
The terminology used for LN in this group of patients has been challenging. The term “seronegative lupus nephritis” has been used to describe patients in whom the renal histology is typical of LN, yet there is no evidence, either clinical or serological, of SLE. Gianviti et al. [10] used the term “full-house” nephropathy. Huerta et al. [13] used the term renal-limited “lupus-like” nephropathy. Sharman et al. [16] described a group of patients with seronegative “full-house” GN as C1q nephropathy.
In conclusion, we describe a child with renal-limited “lupus-like” GN who presented with renal pathology consistent with LN and absent extrarenal manifestations and lupus serology. Recent studies have indicated that this disease entity is not benign, yet the exact pathogenesis is still unknown. Treatment of these patients is challenging and currently not standardized. The clinical course and outcome can be similar to typical LN. Most nephrologists and rheumatologists tend to treat these patients with steroids and immunosuppressive regimens (e.g., cyclophosphamide and/or azathioprine) similar to the protocol for known LN. A “full-house” GN in children may be the first symptom of SLE, with the full-blown picture of the disease delayed by several years. Precise criteria for diagnosis and treatment are still needed.
Statement of Ethics
The authors have no ethical conflicts to declare.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Lech M, Anders H. The pathogenesis of lupus nephritis. J Am Soc Nephrol. 2013;24:1357–1366. doi: 10.1681/ASN.2013010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weening J, D’Agati V, Schwartz M, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich U, Mayer G, Herold M, Klotz W, Stempfl Al-Jazrawi K, Lhotta K. Sensitivity and specificity of autoantibody tests in the differential diagnosis of lupus nephritis. Lupus. 2009;18:1276–1280. doi: 10.1177/0961203309345753. [DOI] [PubMed] [Google Scholar]
- 4.Arbuckle M, McClain M, Rubertone M, Scofield R, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 5.Han S, Zhuang H, Shumyak S, Yang L, Reeves W. Mechanisms of autoantibody production in systemic lupus erythematosus. Front Immunol. 2015;13:228. doi: 10.3389/fimmu.2015.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maddison P, Provost T, Reichlin M. Serological findings in patients with “ANA-negative” systemic lupus erythematosus. Medicine. 1981;60:87–94. doi: 10.1097/00005792-198103000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Reichlin M. ANA negative systemic lupus erythematosus sera revisited serologically. Lupus. 2000;9:116–119. doi: 10.1191/096120300678828091. [DOI] [PubMed] [Google Scholar]
- 8.Coben C, Spizziri F, Drut R. Membranous nephropathy and seronegative lupus erythematosus. Pediatr Nephrol. 2003;18:202–203. doi: 10.1007/s00467-002-1017-9. [DOI] [PubMed] [Google Scholar]
- 9.Jones E, Magil A. Nonsystemic mesangiopathic glomerulonephritis with “full house” immunofluorescence: pathological and clinical observation in five patients. Am J Clin Pathol. 1982;78:29–34. doi: 10.1093/ajcp/78.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Gianviti A, Barsotti P, Barbera V, et al. Delayed onset of systemic lupus erythematosus in patients with “full-house” nephropathy. Pediatr Nephrol. 1999;13:683–687. doi: 10.1007/s004670050681. [DOI] [PubMed] [Google Scholar]
- 11.Baskin E, Agras P, Menekşe N, Ozdemir H, Cengiz N. Full-house nephropathy in a patient with negative serology for lupus. Rheumatol Int. 2007;273:281–284. doi: 10.1007/s00296-006-0198-0. [DOI] [PubMed] [Google Scholar]
- 12.Wen Y, Chen M. Clinicopathological study of originally non-lupus “full-house” nephropathy. Ren Fail. 2010;329:1025–1030. doi: 10.3109/0886022X.2010.510614. [DOI] [PubMed] [Google Scholar]
- 13.Huerta A, Bomback A, Liakopoulos V, Palanisamy A, Stokes MB, D’Agati VD, Radhakrishnan J, et al. Renal limited ‘lupus-like’ nephritis. Nephrol Dial Transplant. 2012;27:2337–2342. doi: 10.1093/ndt/gfr663. [DOI] [PubMed] [Google Scholar]
- 14.Caltik A, Demircin G, Bülbül M, Erdogan O, Akyüz SG, Arda N. An unusual case of ANA negative systemic lupus erythematosus presented with vasculitis, long-standing serositis and full-house nephropathy. Rheumatol Int. 2013;33:219–222. doi: 10.1007/s00296-010-1540-0. [DOI] [PubMed] [Google Scholar]
- 15.Ruggiero B, Vivarelli M, Gianviti A, Pecoraro C, Peruzzi L, et al. Outcome of childhood-onset full-house nephropathy. Nephrol Dial Transplant. doi: 10.1093/ndt/gfw230. DOI: 10.1093/ndt/gfw230. [DOI] [PubMed] [Google Scholar]
- 16.Sharman A, Furness P, Feehally J. Distinguishing C1q nephropathy from lupus nephritis. Nephol Dial Transplant. 2004;19:1420–1426. doi: 10.1093/ndt/gfh139. [DOI] [PubMed] [Google Scholar]