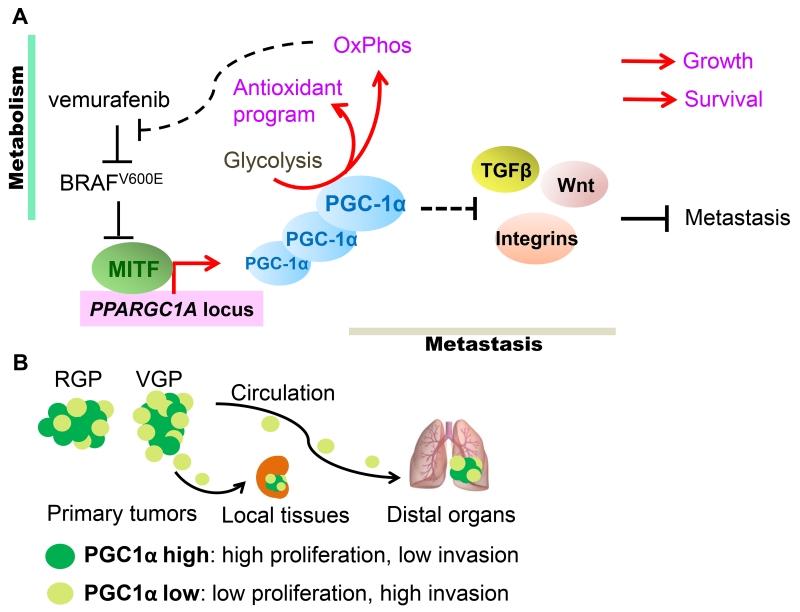
Figure 3. The dualistic nature of PGC-1α in melanomas.
(A). In melanomas, expression of PGC-1α is regulated by the melanocyte master-transcription factor MITF, whose expression in turn is suppressed by signaling downstream of oncogenic NRAS and BRAFV600E. In addition to promoting OxPhos, heightened PGC-1α expression also induces ROS detoxification that ensures cellular survival. Furthermore, in response to BRAF-targeted therapies, such as vemurafenib, mitochondrial biogenesis affords metabolic survival benefits to melanoma cells. However, PGC-1α also attenuates the expression of integrins, TGFβ and Wnt components involved in metastatic dissemination. Consequently, PGC-1α promotes melanoma growth and survival, but through regulation of parallel acting transcriptional programs, also suppresses metastatic spread. (B). Melanoma tumors contain subpopulations of PGC-1α-high and -low cells. The proliferative PGC-1α-high cells are supported by oxidative metabolism, while the PGC-1α-low cells display increased metastatic ability. Specifically, radial growth melanomas (RGP) tend to express more PGC-1α while the invading vertically growth melanomas (VGP) have lessα. These PGC-1α-low cells are endowed with the capacity to locally invade and/or disseminate to circulation and seed distal organs, however once seeded, increased PGC-1α expression facilitates their growth.