Abstract
Many reports show that repeated cocaine administration increases dendritic spine density in medium spiny neurons of the nucleus accumbens, but there is less agreement regarding the persistence of these changes. In this review we examine these discrepancies by systematically categorizing papers that measured cocaine-induced changes in accumbal spine density. We compare published reports based on withdrawal time, short versus long duration of cocaine administration, environmental pairing with cocaine, and core/shell subregion specificity. Together, these studies suggest that cocaine exposure induces rapid and dose-dependent increases in spine density in accumbens neurons that may play a role in the maintenance of cocaine use and vulnerability to early relapse, but are not a factor in behavioral changes associated with longer abstinence.
Introduction
For over a decade, numerous studies have reported increases in dendritic spine density in certain brain areas following cocaine exposure, but the data across studies are not always consistent. Most studies examine morphological changes in the medium spiny neurons (MSNs) of the nucleus accumbens given its role as an important hub in neural networks regulating motivated behavior. The dendritic spines on these MSNs receive excitatory glutamatergic input from multiple cortical and subcortical regions, and this information is integrated with dopaminergic input that can be enhanced by drugs of abuse such as cocaine. Robinson and Kolb [1] initially reported increases in dendritic spine density on MSNs a few weeks after 20 days of intraperitoneal (ip) cocaine injections. This led investigators to posit that spines may mediate long-lasting behavioral changes such as locomotor sensitization or vulnerability to relapse in cocaine addiction. In contrast to this initial report, not all researchers observe persistent cocaine-induced spines after late withdrawal times [2–6]. More recent studies also suggest that cocaine-induced spines can form very early after withdrawal from repeated cocaine injections (30 min to 4 hours) [7,8], and even form after 6 hours – but not 30 min – after a single acute cocaine injection [4,9]
Together, these data suggest that while dendritic spines may form rapidly after cocaine exposure, their duration is more variable, possibly reflecting numerous experimental variables including injection dose, rodent species and strain, sex, and age. Study variables also encompass the dendritic segment measured (proximal or distal), method of spine visualization (Golgi stain, GFP-immunohistochemistry, or diolistic labeling), ip experimenter-administered versus intravenous self-administration, or whether a cocaine challenge dose was administered prior to brain tissue collection. In this brief review, we systematically sorted published findings of cocaine-induced dendritic spines based on four experimental variables to determine whether they would relate to the persistence of dendritic spine changes after cessation of cocaine administration: 1) withdrawal time, 2) days of cocaine administration, 3) whether cocaine was given in the animals’ home cage or in a behavioral test such as locomotor sensitization or conditioned place preference where drugs become associated with specific environmental contexts, and 4) nucleus accumbens subregion (core or shell). The findings suggest a general time course of spine density changes through withdrawal that primarily reflects differences in cocaine treatment duration and subregional analysis, while other variables may have less impact on the duration of spine changes.
Divergent evidence of cocaine-induced spine density changes with longer withdrawal times
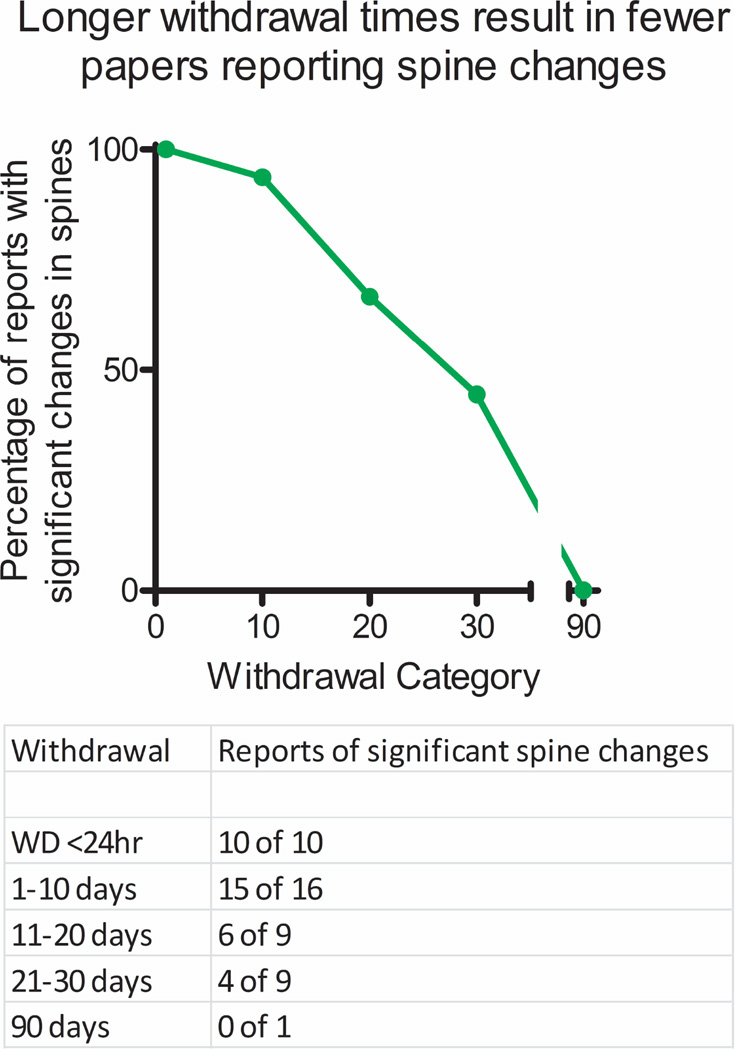
Studies using similar methodology and withdrawal times can find either increases [1], no change [6], or even decreases [5] in spine density in nucleus accumbens neurons. To help rectify these discrepancies, we plotted the percentage of published papers that report a significant change in cocaine-induced spine density for up to 90 days of withdrawal from cocaine treatments. We used the PubMed database with the search terms “accumbens,” “spines,” and “cocaine.” We included every paper (42 in all) that measured either total spine density or changes in subsets of spines (mushroom, thin, stubby, etc.) in cocaine-treated rodents. These papers were then categorized based on 2 criteria: 1) Do the authors report a significant increase in spines (yes or no) and 2) how long after the cocaine treatments was the analysis conducted (< 24 hours, 1–10 days, 11–20 days, 21–30 days, or 31–90 days)? As shown in Figure 1, papers reporting spine density changes at < 24 hours of cocaine treatment all report increases in spine density [5,7,8,10–15]. In fact, it appears that spine increases are evident within 30 min of the final cocaine treatment [8,15] and the minimum cocaine dose reported for these effects with repeated exposure is 10 mg/kg given 5 times over the course of 3 days with only a 4 hour withdrawal period [7]. This suggests that dendritic spine formation reflects an almost immediate response to cocaine exposure, and not a counteradaptive response to prolonged withdrawal from cocaine, as a single acute high dose (30 mg/kg) cocaine injection can produce similar increases in dendritic spine density [4].
Figure 1. Time course of cocaine-induced increases in spine density in nucleus accumbens.
The percentage of studies reporting cocaine-induced spine changes over withdrawal gradually decreases from 100% at early time points (<1 day) to ~ 50% after 1 month. No studies report changes after 3 months of withdrawal. A table reporting the number of positive versus negative reports is given below.
Most papers agree that spine increases can be found at early cocaine withdrawal times from 1 to 10 days [5,6,16–27]. One noteworthy exception is from Smith et al. who observed no changes in wild-type control mice, but did find changes in Fragile × Mental Retardation Protein knockout mice [28]. As the time since withdrawal from cocaine treatments increases, reports on the presence or absence of cocaine-induced spines begin to diverge substantially. After 11–20 days withdrawal, most papers still report spine increases [23,29–33], but some do not [34–36]. However, after 21–30 days after the last cocaine treatment, 5 studies find no increase in spine density [2–6] while 4 studies find that spine increases remain intact [1,20,37,38]. Finally, only one study measured dendritic spines after 90 days withdrawal from cocaine treatments and found no change [39]. Importantly, this negative report incorporated a meaningful positive control where amphetamine-induced spine density increases were still evident after 3.5 months [39]. Together, this body of work suggests an approximate time course for cocaine-induced increases in dendritic spines that rapidly form upon initial cocaine exposure and then gradually decline to normal as early as 1–10 days after withdrawal and are no longer present 30–90 days later.
Some notes on methodology are warranted as papers that incorporated a cocaine challenge after a withdrawal period were excluded [40–43], and most papers reported spine density changes at only one time point. However, some papers assessed multiple time points including Dumitru et al. [5], who measured dendritic spine density at 3 time points and reported changes after 4 hours, 24 hours, and 28 days of cocaine withdrawal. Reports of cocaine-induced spine alterations in transgenic animals were excluded, along with studies reporting spines changes after in utero cocaine exposure (although some show increases in accumbal spine density in later developmental stages [44,45]). This methodology helped ensure a fair and unbiased comparison across published studies.
Longer cocaine dosing regimens may contribute to more persistent spine increases
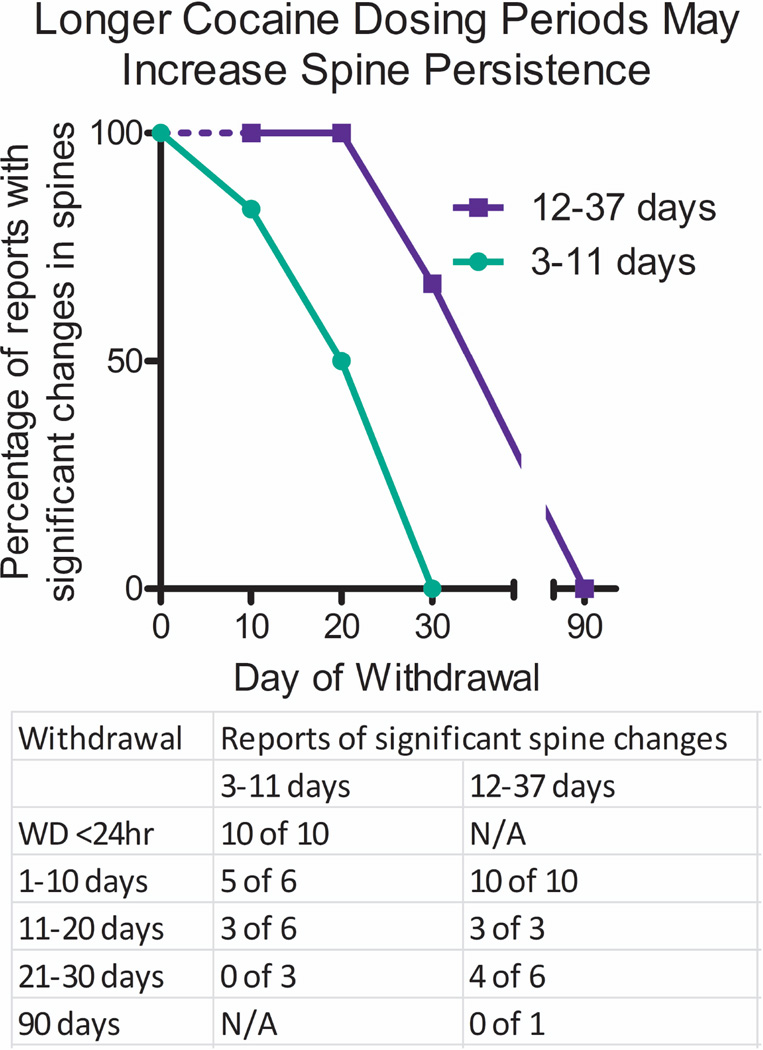
Withdrawal time clearly is a major factor in determining the presence of cocaine-induced spines, but comparisons across multiple studies also suggest that the cocaine-dosing regimen employed can affect the duration of spine increases. Rarely do any two studies use the same treatment durations, and most studies use different daily doses or routes of administration (e.g., ip or iv). Thus, we divided published studies into 2 groups based on either short or long duration of daily treatments. Shorter dosing regimens included 3–11 days of cocaine administration, whereas longer dose regimens incorporated studies that employed 12–37 days of daily cocaine treatments. As shown in Figure 2, all papers with shorter cocaine treatment regimens report significant increases in spines after < 24 hours withdrawal [5,7,8,10–15]. From 1–10 days after withdrawal from cocaine, most of these short treatment studies report that cocaine-induced dendritic spines are still evident in nucleus accumbens MSNs [5,16–18], but Smith et al. found no changes [28]. Increased spines are observed from 11–20 days of withdrawal in at least three reports using short duration cocaine treatments [29–31], but three other studies found no increases in spine density in this time frame [34–36]. Importantly, no study employing the shorter cocaine treatment duration found significant changes in dendritic spine density after 21–30 days after withdrawal [2–4]. In contrast, longer cocaine treatment regimens are more likely to find increased spines at every time point examined, including all reports examining 1–10 days [6,20–27] and 11–20 days after cocaine withdrawal [23,32,33]. Even 21–30 days following longer cocaine treatment regimens, 4 studies report significant increases in cocaine-induced spines [1,20,37,38] but 2 do not [5,6]. At this same withdrawal time, none of the shorter treatment duration groups find significant changes in dendritic spines. Thus, it is clear that longer cocaine treatment regimens lead to more stability in dendritic spines, despite the fact that both short and long cocaine treatments induce robust spine formation at early time points. Finally, the only spine analysis conducted after 90 days employed a long duration cocaine treatment regimen and found no changes as discussed above [39], suggesting that even longer cocaine treatments may not extend the duration of spine changes past 1 month. It is possible that the morphological characteristics of more persistent dendritic spines differ from those that decline early, although this has not been systematically investigated. From this comparison it should be clear that much discrepancy between studies in the literature can be attributed to differences in the duration of cocaine treatment regimens. Shorter cocaine treatment regimens induce spine changes that taper off quickly and are lost after 1 month, while longer dosing regimens decline more slowly and are often present 1 (but not 3) months after cocaine withdrawal.
Figure 2. Short- and long-term cocaine administration periods differentially affect the persistence of cocaine-induced spine density increases in nucleus accumbens.
Reports of cocaine-induced spine density changes were grouped into short-term (3–11 days, green) and long-term (12–37 days, blue) days of cocaine treatment. A dashed blue line indicates no long-term dosing study examined time points at <24 hours of cocaine withdrawal. A table reporting the number of positive versus negative reports is given below.
The environmental context of cocaine administration does not account for discrepancies in the duration of dendritic spines in the nucleus accumbens
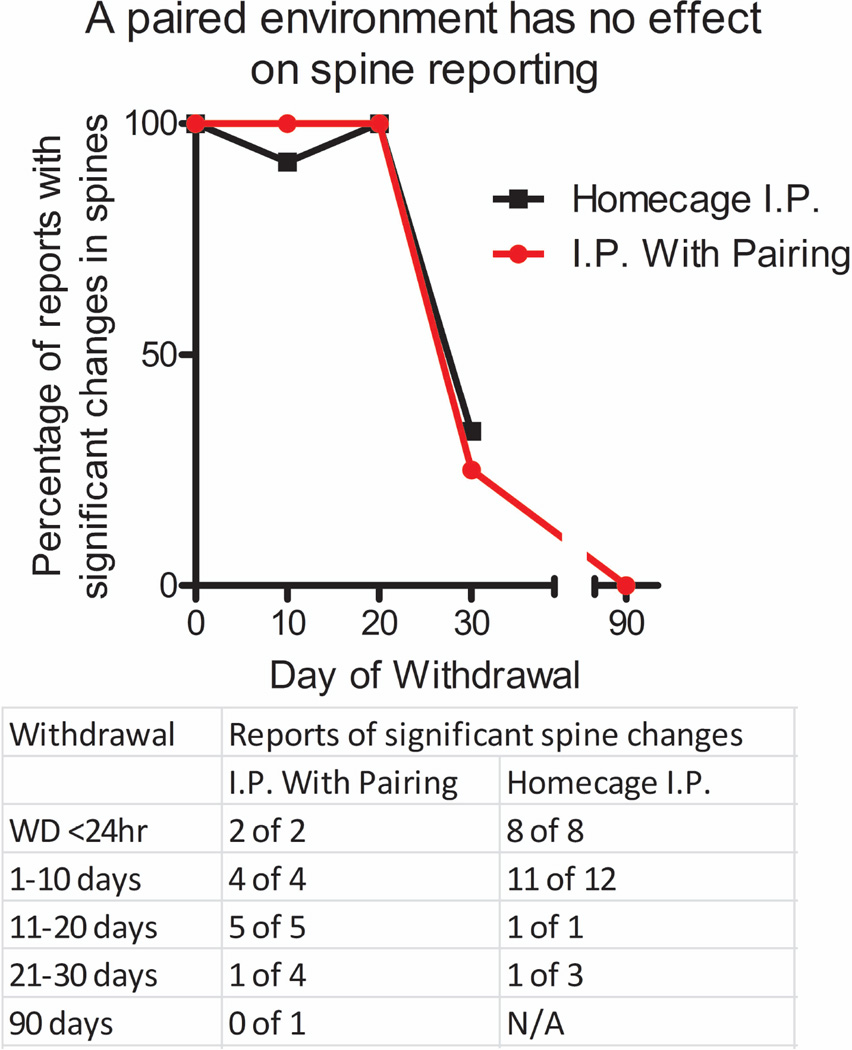
Many neurobiological effects of cocaine administration can be enhanced when cocaine is delivered in a novel or separate cocaine-paired environment that is distinct from the animals’ home cage environment. Indeed, one study found that cocaine-induced spine formation in the nucleus accumbens core subregion will occur with lower doses when cocaine is administered in a distinctive cocaine-paired environment (locomotor test chamber), while changes in the shell subregion occur regardless of the environmental context [29]. Therefore, we compared the duration of dendritic spines in nucleus accumbens MSNs across studies that administered cocaine in the home cage [4,5,7,8,10–14,18–26,28,29] versus those that administered cocaine in a distinctive cocaine-paired environment [1–3,6,15–17,27,29–31,33,39]. The paired group consisted of studies that used locomotor testing apparati, one study that utilized a partially-paired conditioned place preference test chamber[16], and a single study that paired injections with novel, clean scented cages [15]. As shown in Figure 3, there is no clear effect of cocaine-paired versus home cage environments on the percent of studies that find dendritic spine changes over time after withdrawal from the cocaine treatments. This same lack of effect occurs when separating these data by dose between the different environmental contexts (data not shown). Thus, while novel and distinctive cocaine-paired environments may augment the induction of spines with low cocaine doses [29], it does not appear to influence the persistence of spines once they have formed.
Figure 3. Evidence that cocaine-induced changes in spine density in nucleus accumbens is independent of environmental context.
Reports of cocaine-induced spine density changes were grouped based on home cage cocaine treatments or treatments in a distinct cocaine-paired environment. There is no relationship between the cocaine treatment environment and the longevity of spine density increases in published reports. A table reporting the number of positive versus negative reports is given below.
In studies where locomotor sensitization was measured, there is some correlation with the duration of cocaine sensitization and the duration of dendritic spine increases in the nucleus accumbens. Thus, locomotor sensitization appears within a few cocaine injections along with the appearance of spines [15], and both spines and sensitization are no longer present at late time points after cocaine treatments [39]. However, other reports have shown dissociations between spine density increases in the nucleus accumbens and sensitization with both cocaine [28,30] and amphetamine [46]. Together these reports suggest that these behavioral and morphological changes are dissociable.
Dendritic spines in cocaine self-administering animals
While most studies have utilized experimenter-administered ip cocaine injections, some have assessed dendritic spine density changes as a consequence of intravenous cocaine self-administration and withdrawal. There are similar discrepancies in the persistence of spines across these studies. Thus, 3 papers found no increase in dendritic spine density after 11–20 days of withdrawal from chronic cocaine self-administration [34–36], while 3 other papers report significant increases after either 11–20 or 21–30 days of withdrawal [32,37,38]. In papers that report no changes in spine density, animals self-administered cocaine for a minimum criterion of at least 10 days [34–36]. In contrast, spine increases are reported in studies with a higher criterion and generally more days of self-administration: 12–24 days [32], 21–26 days [38], or 28–37 days [37]. As found with the duration of all cocaine regimens discussed above (Figure 2), a longer duration of cocaine self-administration seems to result in more evidence of spine increases, including longer (6 hours) daily access over a similar number of days as shorter (1 hour) daily access [38]. In this regard, more prominent dendritic spine formation is associated with hallmark addicted behavioral profiles in long access animals such as increased cocaine consumption and cocaine-seeking behavior. Conversely, dendritic spine increases ultimately diminish in cocaine withdrawal, whereas cocaine-seeking behavior reportedly increases over similar time frames after cocaine withdrawal [47], suggesting they do not contribute to the incubation of cocaine-seeking behavior. Another possible reason for discrepant findings across studies could be the inclusion of extinction training prior to spine measurements. Thus, 3 studies reporting no change in spine density included 14 days of extinction training after cessation of cocaine self-administration [34–36], while 3 studies that reported spine increases had either no extinction [37,38] or only 10 days of extinction training [32]. Given that extinction training can alter the neurobiological consequences of chronic cocaine self-administration [48,49], it would be interesting to determine the effects of extinction on spine density in future studies. Based on differences in self-administration studies discussed here, it appears that spine formation and/or duration could be influenced by duration of self-administration (Figure 2), the presence of extinction, and/or some interaction between these two variables.
Nucleus accumbens subregions account for some differences in the durations of spines
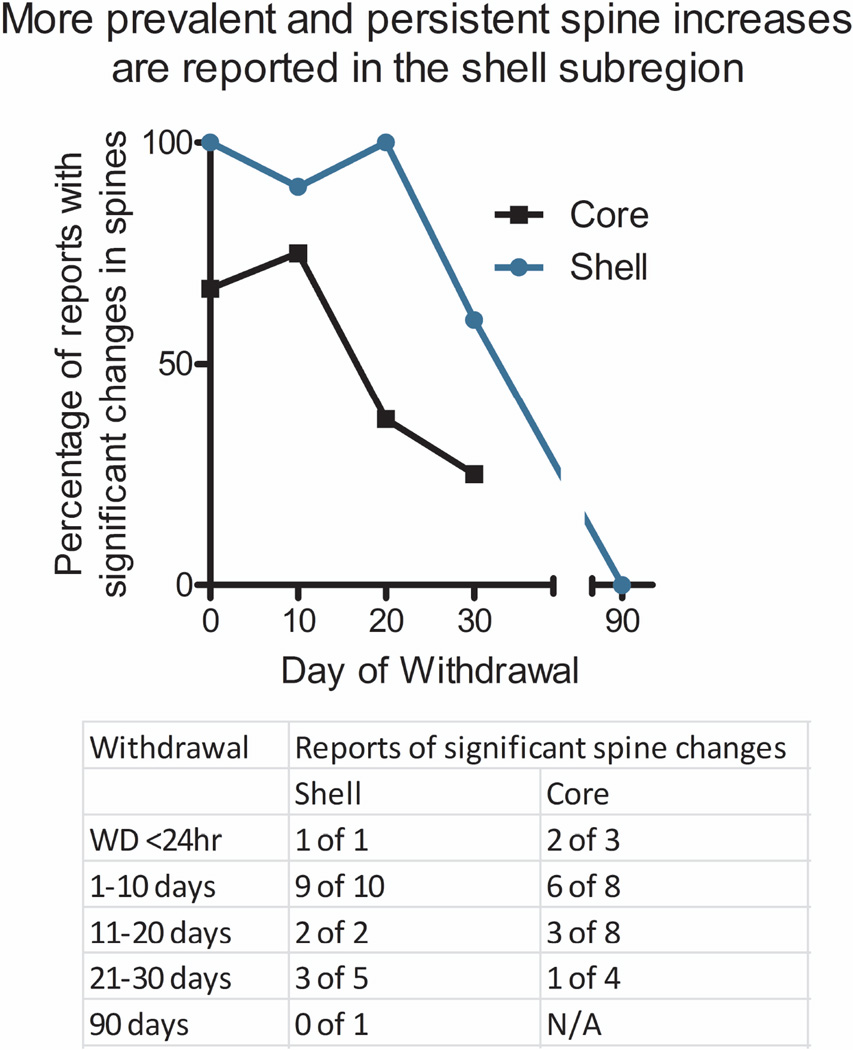
While not all studies examined nucleus accumbens subregions, we compared the duration of dendritic spines in core and shell based on studies that measured spines in one or both subregions separately. For this analysis, studies that examined the accumbens as a whole were excluded and other papers such as Dumitru et al. [5] are highly represented as they reported changes in both the core and shell at 3 different time points. We also counted a study that found significant changes in the core but only a trend (p = 0.055) in the shell as positive reports for both subregions [33]. As shown in Figure 4, a higher percentage of published papers report increases in cocaine-induced dendritic spines in the shell subregion after cocaine withdrawal across all time points when compared to the core subregion. This difference is apparent even at very early withdrawal times, including < 24 hours after the last cocaine exposure. Interestingly, this comparison supports the idea that dendritic spines in the nucleus accumbens shell are more easily induced than core spines by lower doses of cocaine [29]. It also shows that cocaine-induced changes in spine density are more likely to be observed, and longer lasting, in the shell than in the core subregion.
Figure 4. Cocaine-induced spine density changes are found more often in the accumbens shell.
Changes in the shell (blue line) were reported more often than changes in the core (black line) at all time points, including <24hrs. A table reporting the number of positive versus negative reports is given below.
Conclusions
There are several caveats and potential biases with our comparisons that should be considered. Inherent in these comparisons is the idea that spines in the process of reverting to their basal spine densities are more difficult to detect despite their potentially persistent nature, and various methodologies can differ in their ability to measure such changes in size or density. For example, spine density changes absent after one month in one study may be detectable in another study using a different anatomical labeling method (e.g., Golgi, GFP, diolistic labeling) [5,26,50]. Our comparison involves the presence or absence of reported changes and not the degree to which changes in dendritic spine density occur. This approach is heavily weighted to laboratories that publish multiple studies on spine density over those with fewer reports, and positive results are more likely to be published. These caveats would skew results in favor of more persistent changes illustrated in the comparisons we have shown here.
Furthermore, specific nucleus accumbens cell types could factor into these differences, as several papers report that only D1 receptor-containing neurons have increases in spine density with lower doses and duration of cocaine exposure [17,19]. High doses of cocaine result in increased spines in both D1- and D2-receptor containing cells during early withdrawal [23,26], but remain only in D1 neurons after 30 days of withdrawal [20]. However, another study found no spine changes in D1 receptor-containing neurons after 30 days withdrawal [6], suggesting that caveats of dose, duration, and subregion discussed above may extend to these cell-specific measurements. Many other factors undoubtedly play a role since withdrawal time, dosing, and subregion do not account for all the discrepancies in published literature.
Given the positive association between the number of days of cocaine administration and longevity of spines, one possibility is that the duration of spine alterations is related to the strength and duration of neuronal stimulus. Cocaine increases neuronal activation of MSNs and this may lead to a relatively normal cellular response of increased spine density. However, the longer the stimulus is repeated (number of cocaine days), the longer the effect persists, perhaps reflecting the accumulation of other neuroadaptations over time (e.g. [51]). If this is the case, other situations that increase accumbens activity may lead to similar increases in spines. For example, stress can increase dendritic spines in the accumbens when animals are subjected to more aggressive stress regimens such as chronic social defeat stress [52], while weaker stress exposure protocols such as chronic restraint have no effect on dendritic spines in the accumbens [9]. Even milder chronic social defeat stress for 3 sessions of 5 min induce more transient thin spines, while 10 sessions of 10 min cause increases in stubby spine density [52]. Together, these studies suggest that dendritic spine formation is a natural neuronal response of MSNs to activation but longer repetitive periods of enhanced neuronal activity, whether by stress or drugs of abuse, produce more persistent changes.
In summary, our comparison of the duration of cocaine-induced dendritic spines in the nucleus accumbens from published work leads to the following conclusions. First, cocaine-induced increases in spine density can be observed within 1–3 days of the first exposure to cocaine, but they begin to dissipate over the course of 1 month and are no longer present after 3 months. A higher number of days of cocaine administration preserve such spine changes for a longer duration after cocaine withdrawal. In addition, there is no relationship between the persistence of spine density changes in the nucleus accumbens and the environmental context, drug-paired or home cage, of cocaine administration. Finally, these spine changes are clearly more evident in the nucleus accumbens shell than in the core subregion after both early and late withdrawal times. These comparisons illustrate several factors that influence the induction and persistence of dendritic spines and can serve as a useful resource for future investigation of morphological changes that occur in nucleus accumbens MSNs as a result of cocaine use and addiction.
Highlights.
Accumbal dendritic spine changes are observed within 1–3 days of the 1st cocaine use.
3–11d of cocaine leads to short-lived spine increases that are lost after 1 month.
12–37d of cocaine leads to spines that may remain after 1 month of withdrawal.
Spine changes are more prevalent and longer lasting in the shell than the core.
No increases in dendritic spines persist for 90 days after cocaine.
Acknowledgments
We would like to thank Anne Marie Wissman and Dan Guzman for their helpful comments on graphical presentations, and Keitha McCall for her helpful editing.
Funding Sources
This work was supported by the National Institute of Health and National Institute for Drug Addiction (NIDA T32 grant DA 007290 and NIDA DA 08227).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication., As a service to our customers we are providing this early version of the manuscript., The manuscript will undergo copyediting, typesetting, and review of the resulting proof, before it is published in its final citable form. Please note that during the production, process errors may be discovered which could affect the content, and all legal disclaimers, that apply to the journal pertain.
Submission Declaration
The authors declare that this work has not been published anywhere else, was approved by all authors, and will not be published in any other form.
Declaration of Interest
The authors declare no conflicts of interest.
References
- 1. Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. European Journal of Neuroscience. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. The first report of cocaine-induced changes in dendritic spines that can last for weeks.
- 2.Waselus M, Flagel SB, Jedynak JP, Akil H, Robinson TE, Watson SJ., Jr Long-term effects of cocaine experience on neuroplasticity in the nucleus accumbens core of addiction-prone rats. Neuroscience. 2013;248:571–584. doi: 10.1016/j.neuroscience.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toda S, Shen H, Kalivas PW. Inhibition of actin polymerization prevents cocaine-induced changes in spine morphology in the nucleus accumbens. Neurotoxicity Research. 2010;18:410–415. doi: 10.1007/s12640-010-9193-z. [DOI] [PubMed] [Google Scholar]
- 4. Shen H, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. Journal of Neuroscience. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. The report that demonstrates only a single dose of cocaine is needed for a short-lived, transient change in dendritic spine density.
- 5.Dumitriu D, LaPlant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. Journal of Neuroscience. 2012;32:6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. Journal of Neuroscience. 2011;31:1895–1904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dias C, Dietz D, Mazei-Robison M, Sun H, Damez-Werno D, Ferguson D, Wilkinson M, Magida J, Gao V, Neve R, et al. Dishevelled-2 regulates cocaine-induced structural plasticity and Rac1 activity in the nucleus accumbens. Neuroscience letters. 2015;598:23–28. doi: 10.1016/j.neulet.2015.05.003. This paper represents the minimum amount of repeated cocaine injections necessary to see a change in spines: 5 doses of 10mg/kg cocaine ip over 3 days with a 4 hour withdrawal.
- 8.Scobie KN, Damez-Werno D, Sun H, Shao N, Gancarz A, Panganiban CH, Dias C, Koo J, Caiafa P, Kaufman L, et al. Essential role of poly(ADP-ribosyl)ation in cocaine action. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2005–2010. doi: 10.1073/pnas.1319703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esparza MA, Bollati F, Garcia-Keller C, Virgolini MB, Lopez LM, Brusco A, Shen H-W, Kalivas PW, Cancela LM. Stress-induced sensitization to cocaine: actin cytoskeleton remodeling within mesocorticolimbic nuclei. European Journal of Neuroscience. 2012;36:3103–3117. doi: 10.1111/j.1460-9568.2012.08239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. Journal of Neuroscience. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maze I, Covington HE, III, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaPlant Q, Vialou V, Covington HE, III, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iniguez SD, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nature Neuroscience. 2010;13:1137–U1141. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietz DM, Sun H, Lobo MK, Cahill ME, Chadwick B, Gao V, Koo JW, Mazei-Robison MS, Dias C, Maze I, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nature Neuroscience. 2012;15:891–U117. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson D, Koo JW, Feng J, Heller E, Rabkin J, Heshmati M, Renthal W, Neve R, Liu X, Shao N, et al. Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. Journal of Neuroscience. 2013;33:16088–16098. doi: 10.1523/JNEUROSCI.1284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiraly DD, Ma X-M, Mazzone CM, Xin X, Mains RE, Eipper BA. Behavioral and morphological responses to cocaine require kalirin7. Biological Psychiatry. 2010;68:249–255. doi: 10.1016/j.biopsych.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marie N, Canestrelli C, Noble F. Transfer of neuroplasticity from nucleus accumbens core to shell is required for cocaine reward. Plos One. 2012;7 doi: 10.1371/journal.pone.0030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nature Neuroscience. 2014;17:1198–1207. doi: 10.1038/nn.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J, Park B-H, Lee JH, Park SK, Kim J-H. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biological Psychiatry. 2011;69:1026–1034. doi: 10.1016/j.biopsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;119:619–619. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P. Methylphenidate-induced dendritic spine formation and Delta FosB expression in nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2915–2920. doi: 10.1073/pnas.0813179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Li J, Liu N, Wang B, Gu J, Zhang M, Zhou Z, Jiang Y, Zhang L, Zhang L. Signaling via dopamine D1 and D3 receptors oppositely regulates cocaine-induced structural remodeling of dendrites and spines. Neurosignals. 2012;20:15–34. doi: 10.1159/000330743. [DOI] [PubMed] [Google Scholar]
- 25.Ren Z, Sun WL, Jiao H, Zhang D, Kong H, Wang X, Xu M. Dopamine D1 and N-methyl-D-aspartate receptors and extracellular signal-regulated kinase mediate neuronal morphological changes induced by repeated cocaine administration. Neuroscience. 2010;168:48–60. doi: 10.1016/j.neuroscience.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Liu N, Lu K, Zhang L, Gu J, Guo F, An S, Zhang L, Zhang L. Cocaine-induced dendritic remodeling occurs in both D1 and D2 dopamine receptor-expressing neurons in the nucleus accumbens. Neuroscience Letters. 2012;517:118–122. doi: 10.1016/j.neulet.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 27.Martin BJ, Naughton BJ, Thirtamara-Rajamani K, Yoon DJ, Han DD, Devries AC, Gu HH. Dopamine transporter inhibition is necessary for cocaine-induced increases in dendritic spine density in the nucleus accumbens. Synapse. 2011;65:490–496. doi: 10.1002/syn.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith LN, Jedynak JP, Fontenot MR, Hale CF, Dietz KC, Taniguchi M, Thomas FS, Zirlin BC, Birnbaum SG, Huber KM, et al. Fragile X mental retardation protein regulates synaptic and behavioral plasticity to repeated cocaine administration. Neuron. 2014;82:645–658. doi: 10.1016/j.neuron.2014.03.028. A notable exception to most studies examining cocaine-induced changes in spine density at early time points of withdrawal.
- 29.Li YL, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. European Journal of Neuroscience. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Cahill ME, Werner CT, Christoffel DJ, Golden SA, Xie Z, Loweth JA, Marinelli M, Russo SJ, Penzes P, et al. Kalirin-7 mediates cocaine-induced AMPA receptor and spine plasticity, enabling incentive sensitization. Journal of Neuroscience. 2013;33:11012–U11410. doi: 10.1523/JNEUROSCI.1097-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giza JI, Jung Y, Jeffrey RA, Neugebauer NM, Picciotto MR, Biederer T. The synaptic adhesion molecule SynCAM 1 contributes to cocaine effects on synapse structure and psychostimulant behavior. Neuropsychopharmacology. 2013;38:628–638. doi: 10.1038/npp.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-0997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wissman AM, McCollum AF, Huang G-Z, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology. 2011;61:217–227. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stankeviciute NM, Scofield MD, Kalivas PW, Gipson CD. Rapid, transient potentiation of dendritic spines in context-induced relapse to cocaine seeking. Addiction Biology. 2014;19:972–974. doi: 10.1111/adb.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen H-w, Gipson CD, Huits M, Kalivas PW. Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology. 2014;39:1169–1177. doi: 10.1038/npp.2013.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Ferrario CR, Gorny G, Crombag HS, Li YL, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biological Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 39. Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci U S A. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. The end point of cocaine-induced spines. This paper shows no increases in spines density remain after 3 months of withdrawal.
- 40.Gancarz AM, Wang ZJ, Schroeder GL, Damez-Werno D, Braunscheidel KM, Mueller LE, Humby MS, Caccamise A, Martin JA, Dietz KC, et al. Activin receptor signaling regulates cocaine-primed behavioral and morphological plasticity. Nat Neurosci. 2015;18:959–961. doi: 10.1038/nn.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung Y, Mulholland PJ, Wiseman SL, Judson Chandler L, Picciotto MR. Constitutive knockout of the membrane cytoskeleton protein beta adducin decreases mushroom spine density in the nucleus accumbens but does not prevent spine remodeling in response to cocaine. European Journal of Neuroscience. 2013;37:1–9. doi: 10.1111/ejn.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakae DY, Marti F, Lecca S, Vorspan F, Martin-Garcia E, Morel LJ, Henrion A, Gutierrez-Cuesta J, Besnard A, Heck N, et al. The absence of VGLUT3 predisposes to cocaine abuse by increasing dopamine and glutamate signaling in the nucleus accumbens. Mol Psychiatry. 2015;20:1448–1459. doi: 10.1038/mp.2015.104. [DOI] [PubMed] [Google Scholar]
- 43.Chen Q, Xiong X, Lee TH, Liu Y, Wetsel WC, Zhang X. Neural plasticity and addiction: integrin-linked kinase and cocaine behavioral sensitization. Journal of Neurochemistry. 2008;107:679–689. doi: 10.1111/j.1471-4159.2008.05619.x. [DOI] [PubMed] [Google Scholar]
- 44.Salas-Ramirez KY, Frankfurt M, Alexander A, Luine VN, Friedman E. Prenatal cocaine exposure increases anxiety, impairs cognitive function and increases dendritic spine density in adult rats: Influence of sex. Neuroscience. 2010;169:1287–1295. doi: 10.1016/j.neuroscience.2010.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frankfurt M, Wang H-Y, Marmolejo N, Bakshi K, Friedman E. Prenatal cocaine increases dendritic spine density in cortical and subcortical brain regions of the rat. Developmental Neuroscience. 2009;31:71–75. doi: 10.1159/000207495. [DOI] [PubMed] [Google Scholar]
- 46.Singer BF, Tanabe LM, Gorny G, Jake-Matthews C, Li Y, Kolb B, Vezina P. Amphetamine-induced changes in dendritic morphology in rat forebrain correspond to associative drug conditioning rather than nonassociative drug sensitization. Biological Psychiatry. 2009;65:835–840. doi: 10.1016/j.biopsych.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21:RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016;2016:8056370. doi: 10.1155/2016/8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]