Fig. 7.
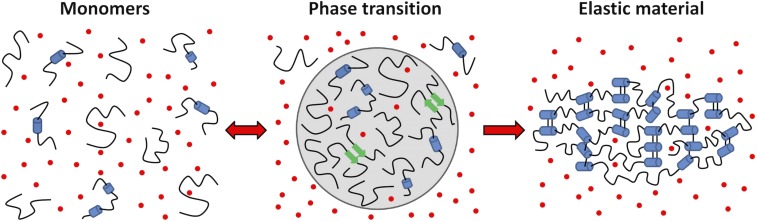
Model for ELP self-assembly. Our findings provide support for a comprehensive model for ELP self-assembly in which elevated temperature and salt increase the entropic cost of solvating hydrophobic chains, driving phase separation. Because the protein sequence precludes adoption of a stable folded state, the resulting phase-separated state is maintained through transient and nonspecific HD interactions between monomers. Whereas the coacervate remains hydrated, bulk water is excluded and there is a reduction in Na+ and Cl− ions (red dots), slightly increasing their concentration in the bulk solution. The coacervated ELP can be chemically cross-linked to form an elastic material. HDs remain dynamic and highly disordered throughout the assembly process, whereas CLD motion slows with each step. Partially helical in the monomers (blue cylinders), CLDs exhibit an increased propensity to form β-sheets (green arrows) in the coacervate and become stable α-helices in cross-linked materials.