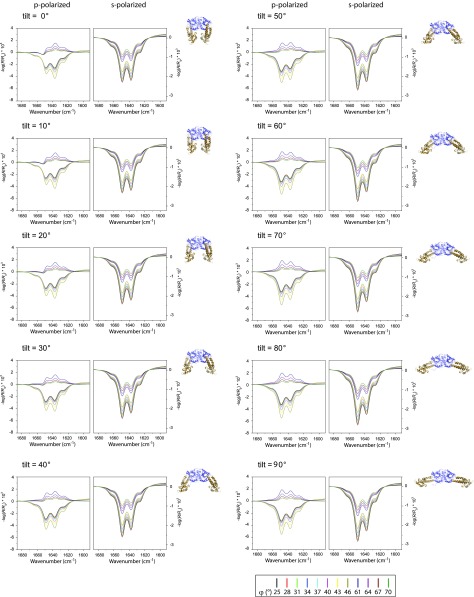
Fig. S5.
IRRA spectra are sensitive to reorientation of the helical domains of EHD2 ΔEH. Calculated IRRA spectra in the amide I′ spectral region for IR light p-polarization (Left) and s-polarization (Right) and various incidence angles φ (see legend). Spectra are calculated for EHD2 ΔEH with systematically increasing tilt angles θ of the helical domains from θ = 0° (crystal structure conformation) to θ = 90° (“open” conformation) as illustrated in the protein sketches (helical domain, gold; G domain, blue) and indicated in the subfigure legends. The orientation of the G domain is constant in all calculations. The helical domain twist angle is ψ = 0°. To reveal the amide I′ band substructure and the effect of conformational change, the spectra are calculated with a reduced bandwidth (fwhm) of their subcomponents (β-sheets, α-helices, random, turns). Note that both band shape and intensity change upon tilting of the helical domain.