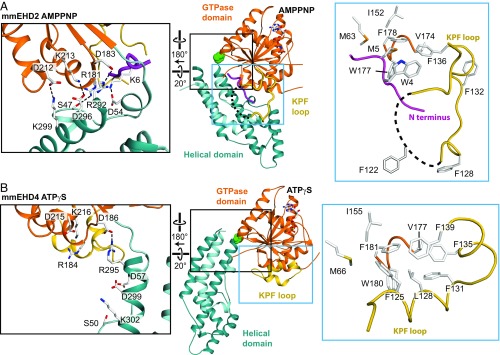
Fig. 3.
The KPF loop undergoes a large-scale reorientation upon activation. (A) Structure of EHD2 (PDB ID code 4CID). The black box highlights the contacts of the helical domain with the GTPase domain and the N terminus in the autoinhibited state. The blue box shows the localization of the N terminus in a hydrophobic groove of the GTPase domain. (B) Structure of EHD4ΔN, with the same orientation of the GTPase domain as in A. The black box features the broken contacts between the helical domain and the GTPase domain in the active state. The blue box shows that the KPF loop occupies the hydrophobic groove in the GTPase domain, with Phe125, Leu128, and Phe131 (corresponding to Phe122, Leu125, and Phe128 in EHD2) acting as anchor points. Because of three extra amino acids in the N-terminal region of EHD4, the numbering of residues on EHD4 (residues 13–541) corresponds to the EHD2 residue number plus three (Fig. S4).