Abstract
A modified twin-screw extruder incorporated with a filtration device was used as a liquid/solid separator for xylose removal from steam exploded corncobs. A face centered central composite design was used to study the combined effects of various enzymatic hydrolysis process variables (enzyme loading, surfactant addition, and hydrolysis time) with two differently extruded corncobs (7% xylose removal, 80% xylose removal) on glucose conversion. The results showed that the extrusion process led to an increase in cellulose crystallinity, while structural changes could also be observed via SEM. A quadratic polynomial model was developed for predicting the glucose conversion and the fitted model provided an adequate approximation of the true response as verified by the analysis of variance (ANOVA).
Keywords: Bioethanol, Lignocellulosic biomass, Mechanical pretreatment
1. Introduction
Bioconversion of lignocellulosic biomass to ethanol is considered to be one of the most important alternatives to petroleum based liquid fuels [14], [15], [17], [29], [35]. Lignocellulosic biomass are highly abundant, have high energy potential and are low cost materials for ethanol production. Typical sources are forest products, agricultural residues, municipal solid waste, and dedicated energy crops [18], [31].
Corncobs, a byproduct of corn grain production, were once used for heat, animal feed and manure for agricultural production in some parts of Europe, while in the United States, corncobs are currently being used as a potential feedstock for cellulosic ethanol production due to its low lignin and high carbohydrate contents. Moreover, corncobs have a high heating value (HHV) producing approximately 8000 Btu/lb. The average corncob yield is about 14% of grain yield, which represents about 16% of the total corn stover in a field [32], [22], [4].
Among the different technologies [25], [33] available for the conversion of lignocellulosic biomass to suitable fermentation substrates, the enzymatic conversion of cellulose seems to be the most promising approach to get a high yield of fermentable sugars [8] because it is highly specific and does not produce substantial amounts of unwanted byproducts [38]. The enzymatic hydrolysis process is usually catalyzed by cellulase enzymes and the process is affected by many factors including cellulose fibre protection by hemicelluloses and lignin, cellulose crystallinity, degree of polymerization, degree of acetylation of hemicelluloses and the accessible surface area of the biomass [28]. The presence of hemicelluloses and lignin makes the cellulase enzymes' access to cellulose difficult, which will reduce hydrolysis efficiency. Therefore, the structure of cellulosic biomass must be pretreated prior to enzymatic hydrolysis to make cellulose more accessible to enzymatic conversion [29], [11]. Various physical, chemical, physico-chemical and biological pretreatment methods have been well-investigated for ethanol production from lignocellulosic biomass [36], [16], [35]. The purpose of the pretreatment is mainly to increase the accessibility of the enzymes to cellulose the by solubilisation of hemicelluloses or/and lignin, and by decreasing the degree of polymerization and cellulose fibre crystallinity [12]. Moreover, adding surfactants has also improved the effectiveness of the cellulose hydrolysis [3], [10].
To improve the rate of enzymatic hydrolysis, researchers have focused on the study of multiple enzymatic hydrolysis process parameters, including substrate concentration, and reaction conditions such as hydrolysis time, pH, temperature and addition of surfactants [35]. Optimal parameters are highly dependant on the physico-chemical structure of the digested biomass, and different pretreatment methods will produce substantially different biomass. Pretreatment in a twin-screw extruder can be used (among other things) to hydrolyze and remove the hemicellulose fraction [23], [24], [7]. However, the effect of xylose removal via extrusion pretreatment, along with other process parameters on the enzymatic hydrolysis of corncobs, has not yet been systematically characterized. In the present study, two differently extruded corncobs with 7% xylose removal and 80% xylose removal, respectively, were used as a source of enzymatic hydrolysis. The characteristics of these two materials were examined by SEM and XRD. A face-centered central composite design was used to study the combined effects of various enzymatic hydrolysis process variables (enzyme loading, surfactant addition, and hydrolysis time) with these two extruded corncobs (7% xylose removal, 80% xylose removal).
2. Materials and methods
Materials
Corncobs were obtained from local farmers in Chatham, ON, Canada. Corncobs were cleaned and ground to the particle size of 0.5–1 cm3 and moisture was adjusted to 50% dry matter. Corncobs were then fed into a continuous steam explosion pretreatment reactor (GreenField Ethanol, Chatham). The reactor was set at a temperature of 205 °C with pH 4.8 in a system pressurized with saturated steam. The overall retention time of the corncobs during pretreatment was 5 min. Hemicellulose was hydrolyzed to xylose or xylo-oligosaccharides under these conditions. The pressure of the reactor was rapidly released to atmospheric pressure, thus the pressurized corncobs were flashed into a cyclone separator, which increased the accessible surface area of the fibres for the enzymes. Pretreated corncobs with 80% moisture content were collected and adjusted to 60% by air drying for further xylose removal during the extrusion process.
All other chemicals (e.g., acetic acid, sodium sulfate anhydrous, tetracycline, cycloheximide, glucose and xylose) were of analytical grade and purchased from Sigma–Aldrich (USA). The Cellic CTec 2 cellulose enzyme was obtained from Novozyme (Canada).
2.2. Xylose removal during extrusion process
Experiments were conducted with a Leistritz co-rotating twin screw extruder (American Leistritz Extruder Corp, USA). The extruder was composed of twelve modular barrels that were each 200 mm long. The barrels were electrically heated using thermal induction and cooled by water circulation. Barrel temperature, water flow rate, feed flow rate and pressure were monitored from a control panel. The material was fed into the extruder inlet port (Barrel 0, Fig. 1) at 4 kg/h by a gravimetric feeder (Brabender Technology, Canada). Water was injected into Barrel 8 by a positive displacement pump (Milton Roy USA). A solid/liquid separator was positioned in Barrel 9 to collect the filtrate mainly containing dissolved xylose. Two pressure sensors were positioned in Barrels 8 and 10, respectively, to detect the pressure on both sides of the filter. Two screw configuration profiles (Fig. 1A and B) were used to produce the extruded corncobs with 7% and 80% xylose removals, respectively. These two screw configuration profiles were built by placing conveying, kneading and reverse screw elements at different positions and intervals. The conveying screw elements were used for material transportation and their smaller pitch could compress the products and achieve a high degree of filling within each barrel. Kneading screw elements oriented at different angles were used to break down large solids and to mix biomass and water to achieve a homogeneous distribution. In addition, reverse screw elements carrying the materials in the opposite direction were placed immediately before and after the filter to increase forward and backward pressure. The only differences between these two screw configuration profiles concerned their backward pressure development zones, situated in zone 11. The backward pressure development zone was composed of two reverse screw elements for Profile A, but only one for Profile B, which caused lower backward pressure, resulting in less xylose removal. All experiments were conducted at a barrel temperature of 100 °C, screw speed of 100 rpm, and a L/S ratio of 1.2.
Fig. 1.
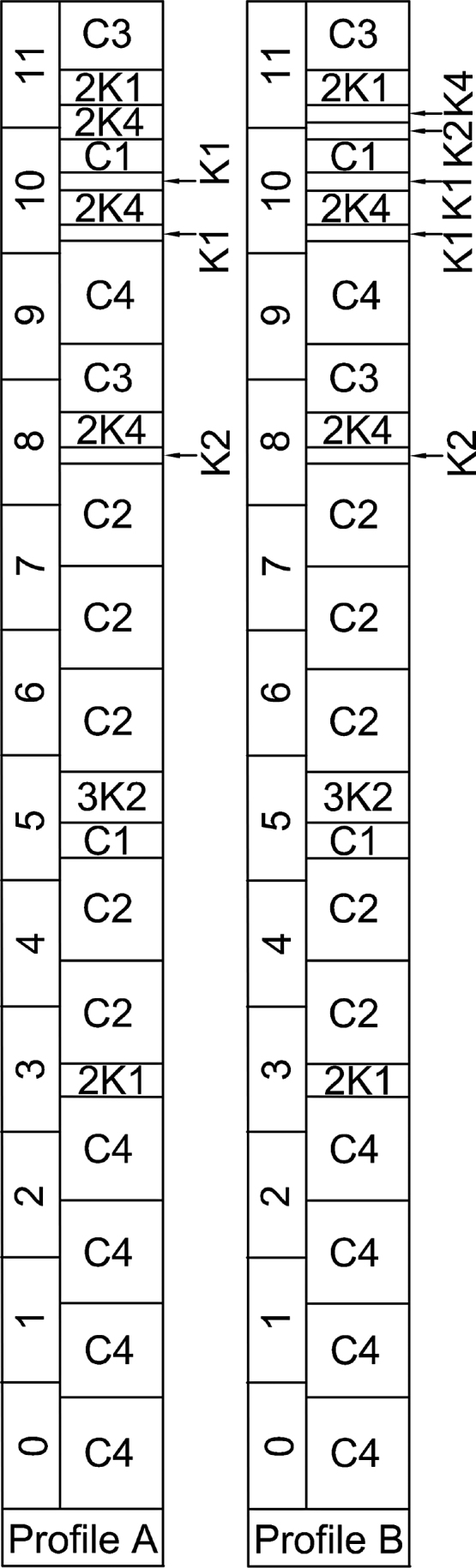
Screw configuration profiles used to achieve 7% (a) and 80% (b) xylose removal. Convening element: C1: GFA-2-30-30, C2: GFA-2-30-90, C3: GFA-2-40-60, C4: GFA-2-40-90. Kneading elements: K1: KB2-15-30°, K2: KB2-15-60°, K3: KB2-15-90°, K4: KB2-15-30°-Li. GFA-2-XX-XX: G = co-rotating, F = conveying, A = free-meshing, 2 = number of threads, the first XX = pitch, the second XX = length of screw element. KB5-2-30-XX-Li: KB = kneading block, 5 = number of kneading segments, 2 = number of threads, 30 = length of kneading block, XX = twisting angle of the individual kneading segments, RE = conveying element, Li = reverse element, X2 = two same elements.
2.3. Carbohydrate analysis
The concentration of glucose was quantified by an Agilent 1260 Infinity high-performance liquid chromatography (HPLC) using a MetaCarb H Plus Column 300 × 7.8 mm (Agilent Technologies, USA), equipped with a refractive index detector. Before analysis, hydrolyzed liquid samples were subjected to 50× dilutions and filtered through a 0.2 μm cellulose acetate membrane (VWR International, USA). The column temperature was maintained at 60 °C and the flow rate was 0.7 ml/min (5 mM H2SO4). The glucose conversion was calculated by comparing the amount of glucose produced in the hydrolyzate to the total amount of glucose monomers present in the pretreated biomass.
2.4. Enzymatic hydrolysis
Enzymatic hydrolysis of extruded corncobs was conducted in 100 ml screw capped glass vials with the Cellic CTec 2 enzyme obtained from Novozyme (Canada). The enzyme activity was measured to be 168.2 FPU/ml. Applied enzyme loadings varied from 1.8 to 7.2 FPU/g DM of the extruded corncobs with 80% xylose removal and from 1.1 to 4.4 FPU/g DM of the extruded corncobs with 7% xylose removal. The enzyme loading was determined based on the total cellulose amount in each extruded corncob. The hydrolysis mixture consisted of 12% (w/v) dry matter/buffer and 0.1 M sodium citrate buffer (pH 5.0), which was supplemented with 40 μl tetracycline and 30 μl cycloheximide to prevent microbial contamination during digestion. Tween 80 (Sigma–Aldrich, USA) was used in these hydrolysis experiments to enhance the enzymatic hydrolysis of extruded corncobs. All vials were incubated at 50 °C in a rotary shaker (Infors HT, Switzerland) at 140 rpm from 48 h to 96 h. Each experiment was conducted in triplicate. 50 μl of an aliquot sample was withdrawn from each reaction mixture at different hydrolysis times according to the experimental design and kept at −20 °C for 10 min to denature enzyme activity. Each sample was diluted, filtered and 1 ml was transferred to a HPLC vial for glucose analysis.
2.5. Scanning electron microscopy (SEM)
The surface properties and microstructure of untreated and pretreated corncob samples were observed using scanning electron microscopy (SEM) (Hitachi S-4800) at an accelerated voltage from 1.0 to 5.0 kV. After air-drying, the surface of the sample was covered with a thin layer of gold before observation using a sputter coater (Emitech K550X, UK) for 3 min to make it more conductive for charge. Digital images were obtained at magnifications ranging from 600× to 20,000×.
2.6. Crystallinity measurement
The crystallinity index is a helpful measure of the relative degree of crystallinity [26], [41]. X-ray diffraction (XRD) was used for phase identification of the untreated and pretreated corncobs. Samples were ground to pass through a 150 μm-mesh screen and the crystallinity was determined by Rigaku (USA) using the CoKα radiation source. Samples were scanned at a speed of 5° (2θ)/min for the continuous run in the 5 to 45° (2θ) range.
The crystalline index (CrI) of cellulose samples was determined through the X-ray diffraction patterns based on the following relationship [6]:
| (1) |
Where Imax represents the maximum intensity peak for cellulose I at 2θ around 26°, Imin represents the minimum intensity peak for the amorphous region (cellulose II) at 2θ around 19° based on Bragg's law conversion from the CuKα radiation source.
2.7. Experimental design
A face centered central composite design (FCCD) with four factors was chosen to evaluate the effect of the selected variables on the response pattern and to determine the optimum combination of enzyme loading (2%–8%), Tween 80 concentration (0%–6%) and hydrolysis time (24 h–72 h) with extruded corncobs with different xylose removals (7% and 80%) were used to maximize glucose conversion from pretreated corncobs. Each factor level was selected based on preliminary studies. Preliminary results from a full factorial design had shown significant curvature (data not shown), hence a central composite design was chosen, in particular, a ‘face centered’ design as only two types of extruded biomass were available (7% and 80% xylose removal). The ratio of the total amount of glucose produced in the hydrolyzate to the total theoretical amount of glucose in the steam-exploded corncobs (analyzed after acid hydrolysis) was chosen as the response for analysis. The experimental design was developed using the software Design Expert, version 8.0.7.1 (Stat Ease, Inc. USA). The resulting 22 experimental conditions, as well as three center point replicates for each type of biomass, were tested in triplicate and data is presented as the average of triplicates ± standard deviation. All experiments were performed fully randomized, and the data was fitted via linear regression to a second order model:
| (2) |
Where y is the predicted response, xi represents the independent variables, k is the number of variables, β0 is the interception coefficient, βi represents the linear coefficient of each independent variable, βii represents the coefficients of the quadratic terms, βij represents the coefficients of the interaction effects and ε is the random error.
Analysis of the variance (ANOVA) was performed and the significance of each variable, the interaction, and quadratic effects were determined based on a significance of α = 0.05 using the F -test. The fitted model was evaluated by R2, adjusted R2, adequate precisior and the lack of fit coefficient for determining the adequacy. In addition, the fitted model was validated by performing experiments using the identified conditions of the significant variables [1].
3. Results and discussion
The carbohydrate composition of the investigated corncobs before and after steam explosion and after different extruder treatments was measured after acid hydrolysis [9], [21], [5]. The data are shown in Table 1 (based on total dry matter). The relative glucose content, which was the largest fraction of monosaccharides, increased from 41% to 66% and 58%, respectively, depending on different extrusion process conditions. The hemicelluloses fraction was largely hydrolyzed to xylose under high temperature and pressure during the steam explosion pretreatment. 7% xylose removal from the steam exploded corncobs was achieved through the extrusion process at a barrel temperature of 65 °C and a screw speed of 100 rpm without adding water, while 80% xylose removal was achieved when the barrel temperature increased to 100 °C and water was injected at Barrel 8 at 2.9 kg/h. Arabinose, galactose, and mannose were found in minor fractions (<5.0%).
Table 1.
Carbohydrate composition of corncob samples after different treatment conditions (average of triplicates ± standard error).
| Ground corncobs (%) | Steam exploded corncobs (%) | Extruded corncobs with 80% xylose removal | Extruded corncobs with 7% xylose removal | |
|---|---|---|---|---|
| Glucose | 41.3 ± 0.75 | 55.4 ± 1.61 | 65.5 ± 1.50 | 58.1 ± 1.52 |
| Xylose | 29.0 ± 1.25 | 22.8 ± 1.29 | 7.4 ± 0.10 | 19.4 ± 1.00 |
| Arabinose | 4.2 ± 0.18 | 2.4 ± 0.07 | 1.5 ± 0.07 | 2.3 ± 0.06 |
| Galactose | 1.5 ± 0.10 | 0.6 ± 0.05 | 0.2 ± 0.03 | 0.6 ± 0.03 |
| Mannose | 0.4 ± 0.03 | 0.3 ± 0.03 | 0.3 ± 0.03 | 0.2 ± 0.02 |
3.1. Biomass characterization
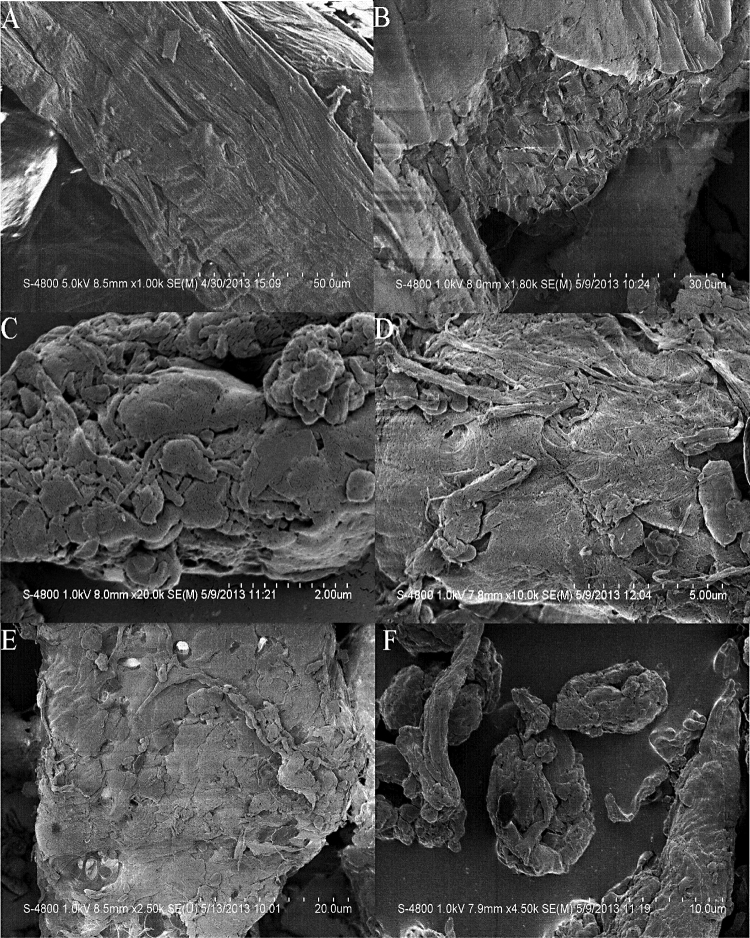
SEM images of untreated and extruded corncobs with different xylose removals at different magnifications are shown in Fig. 2. Based on the differences from SEM micrographs, it was evident that the untreated corncobs exhibited a highly fibrillar, ordered and rigid surface structure and the surface is relatively smooth (Fig. 2A and B); however, after the extrusion pretreatment, the corncobs were separated into differently irregular fibres with different dimensions and some internal areas were fully exposed, thus increasing the internal surface area. At the same time, the surface of extruded corncobs was more chapped, cracked and coarser structures compared to the images in the untreated corncobs. In addition, some pores were observed on the surface of extruded corncobs which could be caused by moisture evaporation under the high temperature (Fig. 2C, D, E and F). Extrusion pretreatment provides mixing, shear force and heat to corncobs; therefore, moisture can evaporate and deeply penetrate corncobs particles during extrusion [40].
Fig. 2.
SEM images at various magnifications for untreated and extruded corncobs. (A,B) untreated corncobs with no xylose removal, (C,D) extruded corncobs with 7% xylose removal, (E,F) extruded corncobs with 80% xylose removal.
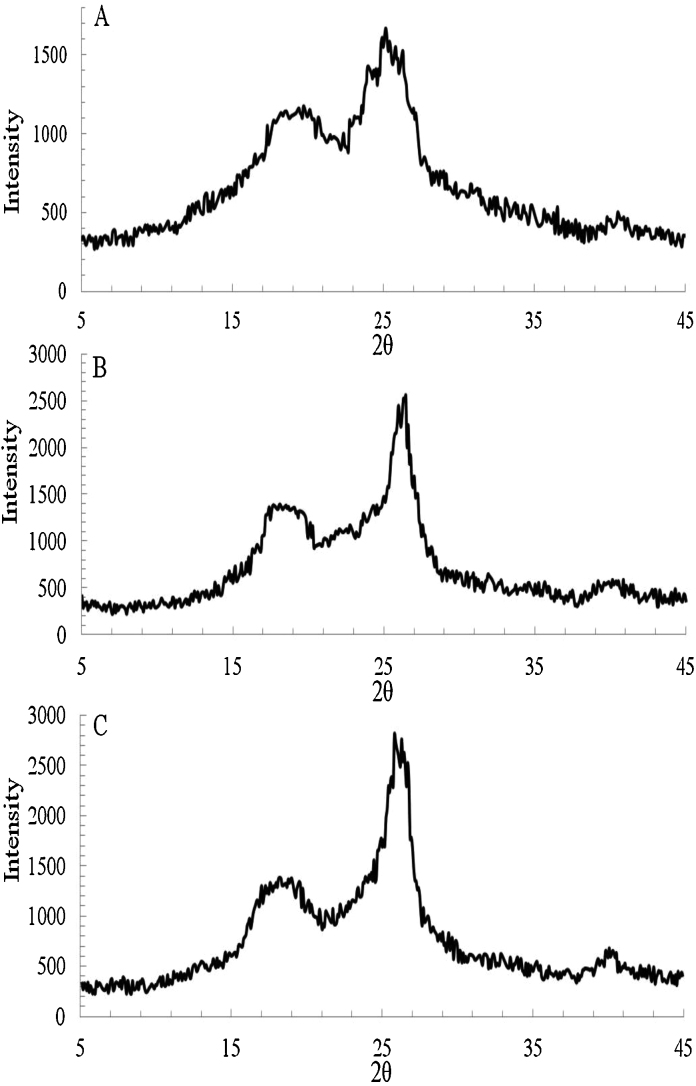
The structures of untreated and extruded corncobs were examined using a powder X-ray diffractometer (XRD) Fig. 3. The crystal structure of cellulose can be changed by various pretreatments by disrupting inter-and intra- chain hydrogen bonding of cellulose fibrils [29]. The diffractogram results show that the untreated and extruded corncobs have the typical cellulose I and cellulose II allomorph characteristics at 2θ = 26° and 2θ = 19°, respectively. For untreated corncobs, the crystalline peak predominates over the amorphous peak, likely due to the presence of higher crystalline cellulose content in untreated corncobs, a form of cellulose which is difficult for enzymatic hydrolysis. The crystallinity index (CrI) for different treatments was calculated from the XRD data by means of three replicates and were 0.304 ± 0.02, 0.462 ± 0.03 and 0.510 ± 0.007 for untreated, ‘7% xylose removed’ and ‘80% xylose removed’, respectively. After the extrusion pretreatment, the peak height of the extruded corncobs increased and became sharper, showing that the amount of cellulose increased, which could be confirmed from the composition analysis in Table 1 and indicates a higher crystallinity degree in the extruded corncobs. The crystallinity increase after pretreatment might be caused by the removal of amorphous components of lignin and hemicelluloses, consistent with values typically reported in the literature. This also confirms that the extrusion pretreatment is an effective method to expose cellulose to enzymatic conversion. An increase in the crystallinity of the extruded corncobs is corresponding to an increase in the rigidity of the cellulose structure, which causes higher tensile strength of fibres [27], [2], [20]. The extruded corncobs with 80% xylose removal have higher cyrstallinity at 2θ = 26° compared to the extruded corncobs with 7% xylose removal, as more amorphous xylose was removed during the extrusion process, thus the content of crystalline cellulose in extruded corncobs with 80% xylose removal is higher than extruded corncobs with 7% xylose removal confirmed in Table 1. This conclusion was also confirmed by the statistical test of analysis of variance (ANOVA) (F value > Fcrit) using Microsoft Office Excel 2007. Sun et al. [34] reported that switchgrass treated with certain ionic liquids increased crystallinity index by reducing amorphous cellulose, hemicelluloses and lignin, resulting in a higher hydrolysis rate by using the Cellic CTec 2 and HTec2. Hall et al. [42] tested the enzymatic hydrolysis rate of the pure cellulosic Avicel and found that the hydrolysis rate increased with a decreasing crystallinity index by endo- and exocellulases. However, the relationship between the crystallinity index of extruded biomass and its corresponding enzymatic hydrolysis rate is not well understood. A biomass with high crystallinity index may not necessarily negatively affect the enzymatic hydrolysis rate [20].
Fig. 3.
X-ray diffraction diagram of untreated and treated corncobs. (A) untreated corncobs with no xylose removal, (B) extruded corncobs with 7% xylose removal, (C) extruded corncobs with 80% xylose removal.
3.2. Enzymatic hydrolysis
The test conditions for enzymatic hydrolysis were chosen based on a statistical experimental design using a face centered central composite design (FCCD). The tested conditions and the resulting glucose conversion are shown in Table 2. The results of the quadratic response surface model are shown in Table 3. The F value of the model is 405.10 which is very high compared to the critical value (2.80), indicating that the model is highly significant. The value of “Prob > F” was less than 0.0001, supporting that the model is significant. The significance of each parameter coefficient was determined by P-values (Prob > F) if their-values were < 0.05. The smaller the P values, the more significant the corresponding coefficient. Among the independent variables, enzyme loading, hydrolysis time, Tween 80 concentration and ‘extruded corncobs with different xylose removals’ had significant effects on glucose conversion. The quadratic effects of enzyme loading and hydrolysis time also had significant effects on glucose conversion. An adjusted R2 of 0.99 confirms the model’s adequacy and no significant lack of fit was detected based on the P value. The signal to noise ratio for all experiments was greater than 4, indicating an adequate signal, which could be used to navigate the design space.
Table 2.
Glucose release from extruded biomass under different conditions, based on central composite design.
| Trial | Factors |
Response |
|||
|---|---|---|---|---|---|
| Enzyme loading (%,w/w) | Tween 80 concentration (%, w/w) | Different xylose removals (%) |
Hydrolysis time (hr) |
Glucose conversion (%) | |
| 1 | 8 | 0 | 80 | 24 | 56.67 ± 1.34 |
| 2 | 5 | 3 | 7 | 48 | 49.87 ± 1.75 |
| 3 | 5 | 3 | 80 | 48 | 61.06 ± 0.73 |
| 4 | 2 | 6 | 80 | 24 | 32.31 ± 2.11 |
| 5 | 8 | 0 | 7 | 24 | 52.18 ± 1.68 |
| 6 | 2 | 0 | 80 | 72 | 38.23 ± 1.31 |
| 7 | 5 | 3 | 80 | 48 | 62.4 ± 0.66 |
| 8 | 2 | 6 | 7 | 24 | 24.3 ± 1.74 |
| 9 | 2 | 0 | 80 | 24 | 26.95 ± 1.02 |
| 10 | 2 | 0 | 7 | 72 | 29.79 ± 1.67 |
| 11 | 8 | 6 | 80 | 24 | 63.94 ± 0.98 |
| 12 | 2 | 6 | 7 | 72 | 31.23 ± 1.25 |
| 13 | 5 | 3 | 7 | 48 | 47.99 ± 1.63 |
| 14 | 8 | 0 | 80 | 72 | 82.03 ± 0.83 |
| 15 | 5 | 3 | 7 | 48 | 46.37 ± 1.80 |
| 16 | 8 | 0 | 7 | 72 | 69.98 ± 1.21 |
| 17 | 2 | 0 | 7 | 24 | 24.15 ± 1.76 |
| 18 | 5 | 3 | 80 | 48 | 62.2 ± 0.85 |
| 19 | 8 | 6 | 7 | 24 | 55.45 ± 1.46 |
| 20 | 2 | 6 | 80 | 72 | 42.83 ± 0.77 |
| 21 | 8 | 6 | 7 | 72 | 72.92 ± 0.91 |
| 22 | 8 | 6 | 80 | 72 | 88.41 ± 0.64 |
| 23 | 5 | 3 | 7 | 24 | 36.57 ± 0.59 |
| 24 | 5 | 3 | 80 | 24 | 45.89 ± 2.05 |
| 25 | 8 | 3 | 80 | 48 | 71.93 ± 1.11 |
| 26 | 2 | 3 | 80 | 48 | 32.38 ± 1.23 |
| 27 | 5 | 0 | 80 | 48 | 54.15 ± 1.15 |
| 28 | 5 | 6 | 80 | 48 | 60.57 ± 1.60 |
Table 3.
Analysis of variance of 2nd order model.
| Source | Sum of squares | Degrees of freedom | Mean square | F value | P value | Remark | |
|---|---|---|---|---|---|---|---|
| Model | 42.35 | 9 | 4.71 | 405.10 | <0.0001 | Significant | |
| Linear | |||||||
| X1 | 31.62 | 1 | 31.62 | 2722.20 | <0.0001 | ||
| X3 | 4.39 | 1 | 4.39 | 377.56 | <0.0001 | ||
| X4 | 3.29 | 1 | 3.29 | 283.48 | <0.0001 | ||
| X2 | 0.34 | 1 | 0.34 | 29.37 | <0.0001 | ||
| Interaction | |||||||
| X1X3 | 0.28 | 1 | 0.28 | 24.08 | 0.0001 | ||
| X3X4 | 0.10 | 1 | 0.10 | 8.80 | 0.0086 | ||
| X2X4 | 0.09 | 1 | 0.09 | 7.65 | 0.0132 | ||
| Quadratic | |||||||
| 0.43 | 1 | 0.43 | 37.41 | <0.0001 | |||
| 0.08 | 1 | 0.08 | 6.84 | 0.0181 | |||
| Residual | 0.20 | 17 | 0.01 | ||||
| Lack of fit | 0.16 | 13 | 0.01 | 1.37 | 0.4111 | Not significant | |
| Pure error | 0.04 | 4 | 9.03 × 10−3 | ||||
| R2 | 0.9954 | ||||||
| Adj-squared | 0.9929 | ||||||
| Pre R2 | 0.9884 | ||||||
| Adequate precisior | 69.64 | ||||||
| C.V. | 1.54 | ||||||
Based on the selected significant variables, the regression analysis yielded the following quadratic model, which was an empirical relationship between glucose conversion and the test variables in terms of coded units (−1 to +1):
| (3) |
Where, Y is the square root of glucose conversion (%); X1, X2, X3 and X4 are enzyme loading, Tween 80 concentration, hydrolysis time and, ‘extruded corncobs with different xylose removals (7%, 80%), respectively.
3.2.1. Combined effects of variables on glucose conversion
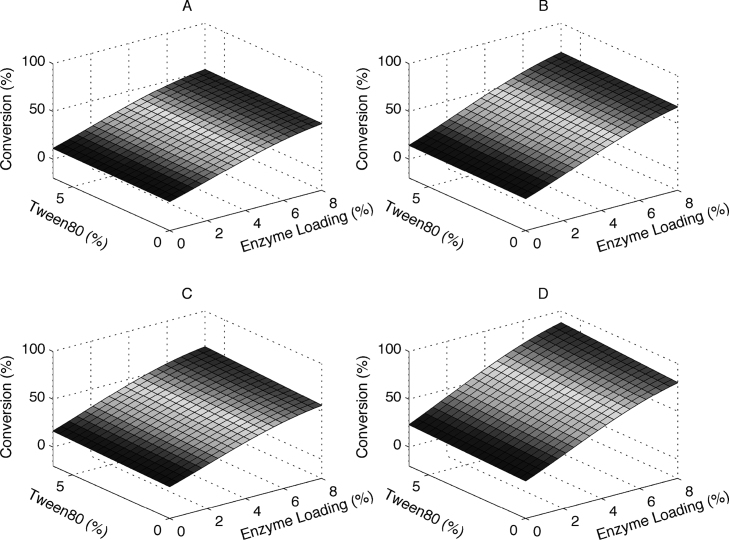
Surface plots were generated to further illustrate the interaction of corresponding parameters. The effect of Tween 80 concentration and enzyme loading on the enzymatic hydrolysis of extruded corncobs is shown in Fig. 4. For the extruded corncobs with 7% xylose removal, as can be seen in Fig. 4A and B, the glucose conversion was not affected significantly in the presence of the Tween 80 when the enzyme loading and hydrolysis time were varied (P = 0.05). This indicates that xylose might be the major factor limiting enzymatic hydrolysis. For the extruded corncobs with 80% xylose removal, the effect of Tween 80 was very small at 24 h (Fig. 4C). However, when the hydrolysis time was prolonged to 72 h (Fig. 4D), increasing Tween 80 concentration resulted in a significant increase in glucose conversion at a high level of enzyme loading (P < 0.05). However as the hydrolysis time increases it would be expected to see a decrease of the hydrolysis rate due to cellulosic substrate decrease, increase of potentially inhibitory end- and by-products and general enzyme deactivation [13]; potentially more evident at low enzyme loadings. The plot shows that a higher hydrolysis yield was obtained in the presence of a high level of Tween 80 concentration. For example, the difference in the glucose conversion was changed from 36% to 42% when the enzyme loading was 2%, and a higher difference was obtained from 80% to 88% when the Tween 80 concentration increased to 6% at an enzyme loading of 8%. In addition, the surfactant also could prevent the unproductive binding of cellulase to lignin by absorbing into the surface of lignin. This enabled the more active enzyme to only react with cellulose to improve the glucose conversion [10].
Fig. 4.
Response surface plot showing interaction effects of Tween 80 concentration and enzyme loading (A, B: constant hydrolysis time of 24 h and 72 h, respectiveley with extruded corncobs with 7% xylose; C,D: constant hydrolysis time of 24 h and 72 h, respectively with extruded corncobs with 80% xylose removal).
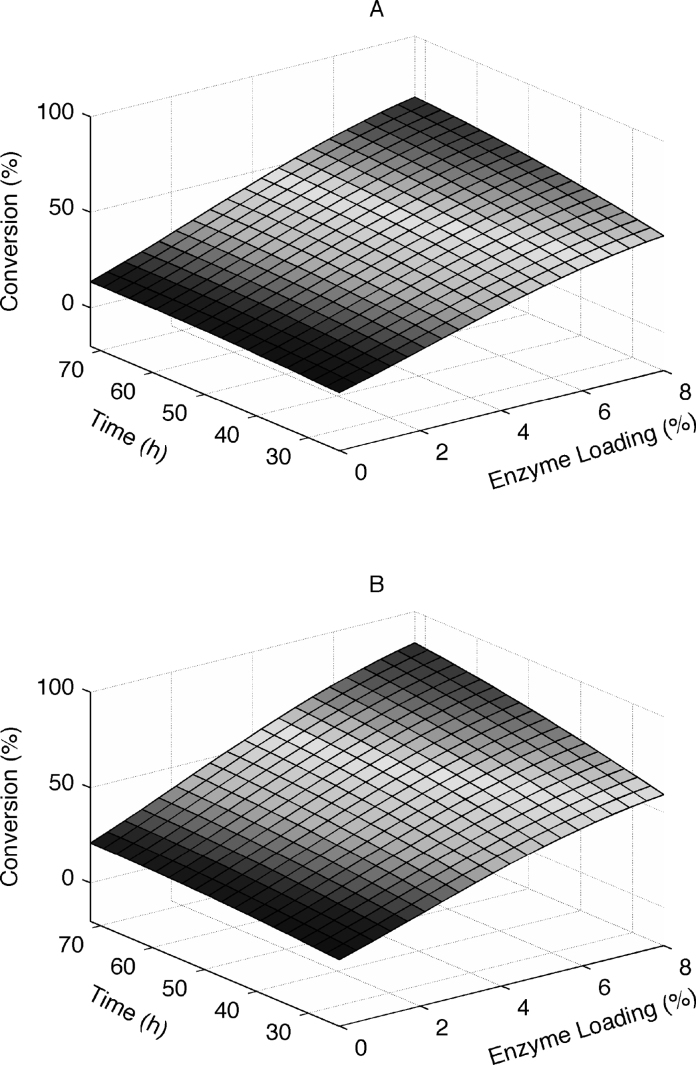
The combined effect of enzyme loading and hydrolysis time at fixed Tween 80 concentration (3%) is shown in Fig. 5. As can be seen from Fig. 5A, the conversion of glucose increased from 22% to 29% at an enzyme loading of 2% with extruded corncobs with 7% xylose removal, but increased from 51% to 68% at 8% enzyme loading when increasing hydrolysis time from 24 to 72 h. The effects of hydrolysis time on the glucose conversion of extruded corncobs with 80% xylose removal were also observed (Fig. 5B). When enzyme loading was at 2%, glucose conversion was only 28% at the hydrolysis time of 24 h. Increasing the amount of cellulase significantly improved the glucose conversion to 59% when enzyme loading increased from 2% to 8%. Enzyme crowding on the cellulose surface, an effect that can result in lower hydrolysis rates at increasing enzyme concentrations [37], was not observed under the experimental conditions. An increase in hydrolysis time from 24 to 72 h at 2% enzyme loading only resulted in a slight increase in the glucose conversion. This might be due to not enough cellulase reaching adsorption saturation for a certain amount of cellulose hydrolysis in the reaction mixture. Further increases in the enzyme loading would slow down the glucose conversion due to more unused cellulase in the mixture solution. Thus, as expected, glucose conversion could be increased with longer hydrolysis times at a higher enzyme loading.
Fig. 5.
Response surface plot of the combined effects of hydrolysis time and enzyme loading on the glucose conversion. (A: constant Tween 80 concentration (3%) with extruded corncobs with 7% xylose removal; B: constant Tween 80 concentration (3%) with extruded corncobs with 80% xylose removal).
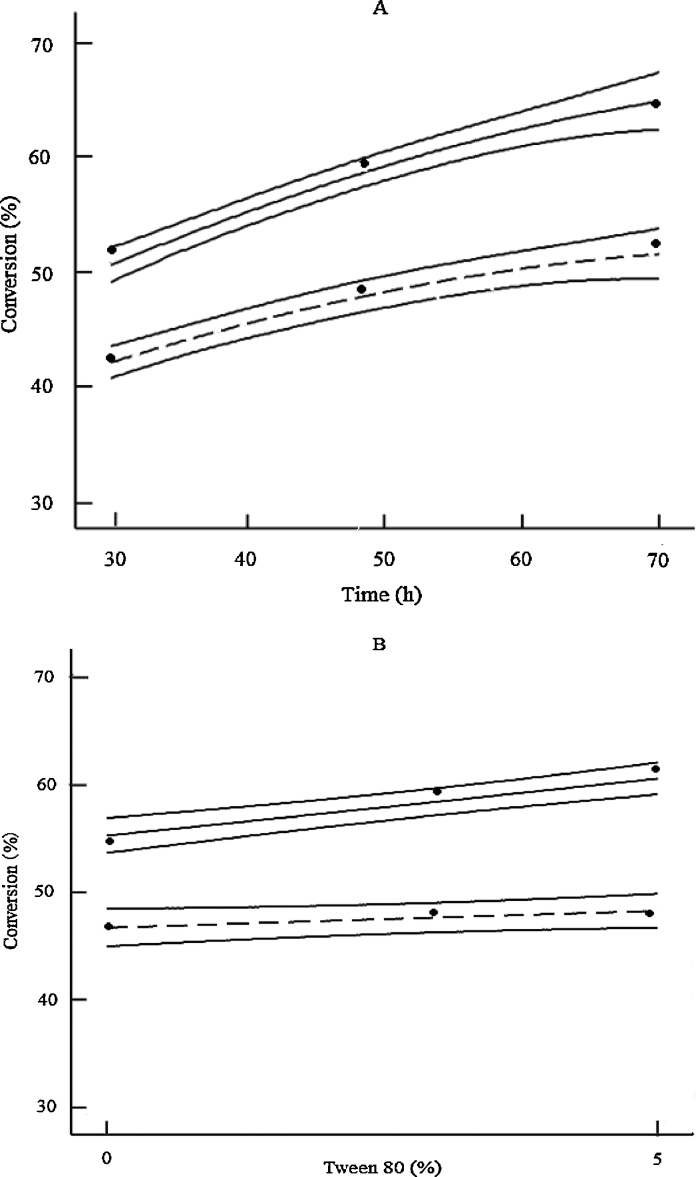
The effect for xylose removal (designated as a categorical parameter) could be visualized in two-dimensional plots as shown in Fig. 6. Fig. 4, Fig. 5 already show that high xylose removal clearly resulted in enhanced enzymatic digestibility. Fig. 6 highlights this by showing the model results for glucose conversion as a function of hydrolysis time and Tween 80 surfactant concentration, respectively, for both types of biomass, while the remaining variables were at their center points. These findings are consistent with several studies showing that cellulose conversion by enzymatic hydrolysis can be facilitated if a high percentage of hemicelluloses are removed [19], [39], [30].
Fig. 6.
2D plot showing interaction effects of extruded corncobs with different xylose removals with hydrolysis time, Tween 80 concentration on the glucose conversion, respectively. (A: constant Tween 80 concentration (3%) with enzyme loading of 5%; B: constant hydrolysis time of 48 h with enzyme loading of 5%; dash line: extruded corncobs with 7% xylose removal; solid line: extruded corncobs with 80% xylose removal. Symbols represent measured data.
3.2.2. Model validation
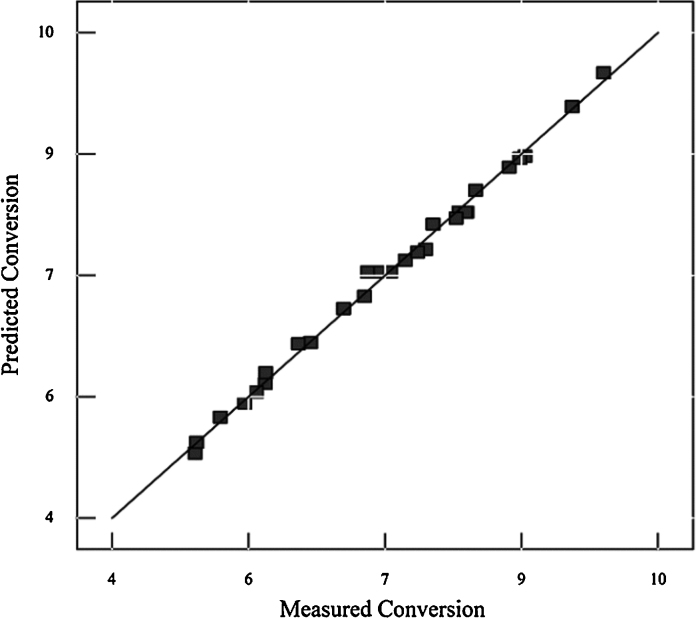
In order to confirm the validity and applicability of the second-order polynomial regression model obtained from the experimental data, six confirmation runs were carried out as listed in Table 4 to compare the difference between the predicted and measured values. The results in Table 4 shows that the difference is below 3%. A plot of predicted versus measured values as shown in Fig. 7 also verifies the overall good fit of the suggested models, indicating that the proposed model could be a useful and accurate model to express the actual relationship between the response and significant variables to predict the glucose conversion.
Table 4.
Operating conditions and predicted and measured response of confirmation experiments.
| Trial | Enzyme loading (%) | Tween 80 concentration (%) |
Different xylose removals (%) | Hydrolysis time (h) | Predicted glucose conversion (%) | Measured glucose conversion (%) | Error |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 6.00 | 80 | 72 | 87.66 | 90.01 ± 0.69 | +2.61 |
| 2 | 5 | 3.00 | 7 | 48 | 47.61 | 48.56 ± 1.85 | +1.96 |
| 3 | 7.2 | 5.90 | 80 | 72 | 83.55 | 85.11 ± 0.90 | +1.83 |
| 4 | 4 | 2.00 | 7 | 60 | 42.94 | 43.70 ± 1.25. | +1.74 |
| 5 | 5 | 3.00 | 80 | 48 | 58.37 | 59.65 ± 1.04 | +2.15 |
| 6 | 2 | 3.00 | 7 | 72 | 28.59 | 29.45 ± 2.09 | +2.92 |
Fig. 7.
Predicted versus measured values for glucose conversion.
4. Conclusion
Twin-screw extruders can be used as a pretreatment method for lignocellulosic biomass to produce material with varying xylose contents. The xylose content can be controlled based on the employed screw configuration, as demonstrated for steam-exploded corncobs. The extrusion process further led to an increase in cellulose crystallinity, while structural changes were also observed via SEM. The effects of residual xylose (7% and 80% removal through extrusion process), enzyme loading, surfactant addition, and hydrolysis time on enzymatic hydrolysis could be described with an 2nd order polynomial model, based on data generated through a face-centered central composite design. All independent variables and the interaction effects of enzyme loading and hydrolysis time, hydrolysis time and xylose content, Tween 80 concentration and xylose content, the quadratic terms of enzyme loading as well as the quadratic term of hydrolysis time had a significant effect on enzymatic hydrolysis.
Acknowledgements
The authors would like to thank the GreenField Specialty Alcohols Inc. (Chatham, Canada), Centres of Excellence for Commercialization and Research (CECR) Canada, Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Foundation for Innovation (CFI) for financial support.
Footnotes
Available online 26 June 2014
References
- 1.Adel A.M., Abd El-Wahab Z.H., Ibrahim A.A., Al-Shemy M.T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresour. Technol. 2010;101:4446–4455. doi: 10.1016/j.biortech.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 2.Alemdar A., Sain M. Biocomposites from wheat straw nanofibers: morphology, thermal and mechanical properties. Compos. Sci. Technol. 2008;68:557–565. [Google Scholar]
- 3.Alkasrawi M., Eriksson T., Börjesson J., Wingren A., Galbe M., Tjerneld F., Zacchi G. The effect of Tween-20 on simultaneous saccharification and fermentation of softwood to ethanol. Enzyme Microb. Technol. 2003;33:71–78. [Google Scholar]
- 4.Barl B., Biliaderis C.G., Murray E.D., Macgregor A.W. Combined chemical and enzymatic treatments of corn husk lignocellulosics. J. Sci. Food Agric. 1991;56:195–214. [Google Scholar]
- 5.Borchardt L.G., Piper C.V. A gas chromatographic method for carbohydrates as alditol-acetates. Tappi. 1970;53:257–260. [Google Scholar]
- 6.Cao Y., Tan H. Study on crystal structures of enzyme-hydrolyzed cellulosic materials by X-ray diffraction. Enzyme Microb. Technol. 2005;36:314–317. [Google Scholar]
- 7.Dottori, F.A., Benson, R.A.C., Benech, R.O., inventors; January 13 2013, Fractionation of lignocellulosic biomass for cellulosic ethanol and chemical production. United States patent US 0017589.
- 8.El-Zawawy W.K., Ibrahim M.M., Abdel-Fattah Y.R., Soliman N.A., Mahmoud M.M. Acid and enzyme hydrolysis to convert pretreated lignocellulosic materials into glucose for ethanol production. Carbohydr. Polym. 2011;84:865–871. [Google Scholar]
- 9.Englyst H., Wiggins H.S., Cummings J.H. Determination of the non-starch polysaccharides in plant foods by gas–liquid chromatography of constituent sugars as alditol acetates. Analyst. 1982;107:307–318. doi: 10.1039/an9820700307. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson T., Börjesson J., Tjerneld F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb. Technol. 2002;31:353–364. [Google Scholar]
- 11.Gao K., Rehmann L. ABE fermentation from enzymatic hydrolysate of NaOH-pretreated corncobs. Biomass Bioenerg. 2014;66:110–115. [Google Scholar]
- 12.Gil N., Ferreira S., Amaral M.E., Domingues F.C., Duarte A.P. The influence of dilute acid pretreatment conditions on the enzymatic saccharification of Erica spp. for bioethanol production. Ind. Crops Prod. 2010;32:29–35. [Google Scholar]
- 13.Gregg D.J., Saddler J.N. Factors affecting cellulose hydrolysis and the potential of enzyme recycle to enhance the efficiency of an integrated wood to ethanol process. Biotechnol. Bioeng. 1996;51:375–383. doi: 10.1002/(SICI)1097-0290(19960820)51:4<375::AID-BIT1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Hahn-Hägerdal B., Galbe M., Gorwa-Grauslund M.F., Lidén G., Zacchi G. Bio-ethanol– the fuel of tomorrow from the residues of today. Trends Biotechnol. 2006;24(12):549–556. doi: 10.1016/j.tibtech.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Hamelinck C.N., Van Hooijdonk G., Faaij A.P.C. Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenerg. 2005;28(4):384–410. [Google Scholar]
- 16.Hendriks A.T.W.M., Zeeman G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009;100:10–18. doi: 10.1016/j.biortech.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Himmel M.E., Ding S.-Y., Johnson D.K., Adney W.S., Nimlos M.R., Brady J.W., Foust T.D. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 2007;315(5813):804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 18.Hsu T.C., Guo G.L., Chen W.H., Hwang W.S. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010;101:4907–4913. doi: 10.1016/j.biortech.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Kabel M.A., Bos G., Zeevalking J., Voragen A.G.J., Schols H.A. Effect of pretreatment severity on xylan solubility and enzymatic breakdown of the remaining cellulose from wheat straw. Bioresour. Technol. 2007;98:2034–2042. doi: 10.1016/j.biortech.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Kim S., Holtzapple M.T. Effect of structural features on enzyme digestibility of corn stover. Bioresour. Technol. 2006;97:583–591. doi: 10.1016/j.biortech.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Krull L.H., Inglett G.E. Analysis of neutral carbohydrates in agricultural residues by gas–liquid chromatography. J. Agric. Food Chem. 1980;28:917–919. doi: 10.1021/jf60231a013. [DOI] [PubMed] [Google Scholar]
- 22.Latif F., Rajoka M.I. Production of ethanol and xylitol from corncobs by yeasts. Bioresour. Technol. 2001;77:57–63. doi: 10.1016/s0960-8524(00)00134-6. [DOI] [PubMed] [Google Scholar]
- 23.Lehoux, R.R., Bradt, C, B., inventors; October 10 2013, Twin screw extruder press for solid/fluid separation. United States patent US 0264264.
- 24.Lehoux, R.R., Bradt, C, B., inventors; May. 17 2012, Solid/fluid separation device and method for treating biomass including solid/fluid separation. United States patent US 0118517.
- 25.Luque L., Westerhof R., Van Rossum G., Oudenhoven S., Kersten S., Berruti F., Rehmann L. Pyrolysis based bio-refinery for the production of bioethanol from demineralized ligno-cellulosic biomass. Bioresour. Technol. 2014;161:20–28. doi: 10.1016/j.biortech.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Lynd L.R., Weimer P.J., Van Zyl W.H., Pretorius I.S. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002;66:506–577. doi: 10.1128/MMBR.66.3.506-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda R.N., Serpa V.I., Rocha V.A.L., Mesquita R.A.A., Anna L.M.M.S., de Castro A.M., Driemeier C.E., Pereira N., Jr, Polikarpov I. Enzymatic hydrolysis of pretreated sugar cane bagasse using Penicillium funiculosum and Trichoderma harzianum cellulases. Process Biochem. 2011;46:1196–1201. [Google Scholar]
- 28.McMillan J.D. Pretreatment of lignocellulosic biomass. In: Himmel M.E., Baker J.O., Overend R.P., editors. Enzymatic Conversion of Biomass for Fuels Production. ACS; Washington DC: 1994. pp. 292–324. [Google Scholar]
- 29.Mosier N., Wyman C., Dale B., Elander R., Lee Y.Y., Holtzapple M., Ladisch M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005;96:673–686. doi: 10.1016/j.biortech.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Palonen H., Thomsen A.B., Tenkanen M., Schmidt A.S., Viikari L. Evaluation of wet oxidation pretreatment for enzymatic hydrolysis of softwood. Appl. Biochem. Biotechnol. 2004;117:1–17. doi: 10.1385/abab:117:1:01. [DOI] [PubMed] [Google Scholar]
- 31.Palonen H., Tjerneld F., Zacchi G., Tenkanen M. Adsorption of trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolatd lignin. J. Biotechnol. 2004;107:65–72. doi: 10.1016/j.jbiotec.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 32.G. Roth, C. Gustafson, Corncobs for biofuel production Unpublished results http://www.extension.org/pages/26619/corn-cobs-for-biofuel-production#cite_note-1 (retrieved 30.1.14.) 2014.
- 33.Schwab K., Wood J.A., Rehmann L. Pyrolysis byproducts as feedstocks for fermentative biofuel production: an evaluation of inhibitory compounds through a synthetic aqueous phase. Ind. Eng. Chem. Res. 2013;52(51):18234–18240. [Google Scholar]
- 34.Sun N., Parthasarathi R., Socha A.M., Shi J., Zhang S., Stavila V., Sale K.L., Simmons B.A., Singh S. Understanding pretreatment efficacy of four cholinium and imidazolium ionic liquids by chemistry and computation. Green Chem. 2014 [Google Scholar]
- 35.Sun Y., Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour. Technol. 2002;83:1–11. doi: 10.1016/s0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 36.Tian S.Q., Wang Z.Y., Fan Z.I., Zuo L.L. Optimization of CO2 laser-based pretreatment of corn stover using response surface methodology. Bioresour. Technol. 2011;102:10493–10497. doi: 10.1016/j.biortech.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 37.Warden A.C., Little B.A., Haritos V.S. A cellular automaton model of crystalline cellulose hydrolysis by cellulases. Biotechnol Biofuels. 2011;4:39. doi: 10.1186/1754-6834-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen Z., Liao W., Chen S. Hydrolysis of animal manure lignocellulosics for reducing sugar production. Bioresour. Technol. 2004;91:31–39. doi: 10.1016/s0960-8524(03)00166-4. [DOI] [PubMed] [Google Scholar]
- 39.Yang B., Wyman C.E. Effect of xylan and lignin removal by batch and flowthrough pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol. Bioeng. 2004;86:88–95. doi: 10.1002/bit.20043. [DOI] [PubMed] [Google Scholar]
- 40.Zhan X., Wang D., Bean S.R., Mo X., Sun X.S., Boyle D. Ethanol production from supercritical-fluid-extrusion cooked sorghum. Ind. Crop Prod. 2006;23:304–310. [Google Scholar]
- 41.Zhang Y.H., Lynd L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol. Bioeng. 2004;88:797–824. doi: 10.1002/bit.20282. [DOI] [PubMed] [Google Scholar]
- 42.Hall M., Bansal P., Lee J.H., Realff M.J., Bommarius A.S. Cellulose crystallinity – a key predictor of the enzymatic hydrolysis rate. FEBS J. 2010;277:1571–1582. doi: 10.1111/j.1742-4658.2010.07585.x. [DOI] [PubMed] [Google Scholar]