Figure 6.
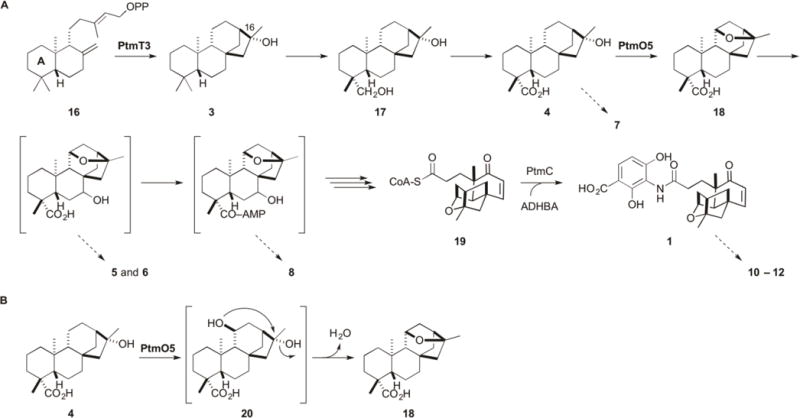
Proposed biosynthesis of PTM supported by the isolation of two intermediates (3 and 4), four congeners (5–8), as well as three fully processed PTM analogues (10–12) from S. platensis SB12036, and intermediates (17 and 18) previously isolated from SB12030.9 (A) ent-CPP (16) is cyclized into (16R)-ent-kauran-16-ol (3) by PtmT3. Oxidation at C-19 likely occurs before 11S,16S-ether ring formation, which is catalyzed by PtmO5. Hydroxylation at C-7 and activation by adenylation at C-19, occurs before A-ring cleavage and β-oxidation. The A-ring is shown in 16. Platensicyl-CoA (19) is coupled to ADHBA by PtmC in the final step of PTM biosynthesis.19 Proposed intermediates in brackets have not been isolated or experimentally confirmed, but are supported by the congeners isolated and indicated with the dotted arrows. Isolation of the adenylated ent-atiserene congener (9) (see Figure 1) also supports parallel processing of the ent-atiserene-derived intermediates in PTN biosynthesis. Congeners 10–12 highlight the flexible nature of the processing enzymes. (B) Proposed model for 11S,16S-ether ring formation by PtmO5. Due to the necessary inversion of stereochemistry at C-16 from 4 to the ether product, an 11S,16R-bis-hydroxylated intermediate (20) is predicted. The final 11S,16S-ether ring formation step may proceed enzymatically as a part of the PtmO5 activity or nonenzymatically as a spontaneous nucleophilic substitution.
