Abstract
In the current study, we demonstrate that the silencing of protein kinase R (PKR)-like endoplasmic reticulum (ER) kinase (PERK) and activating transcription factor 6 (ATF4) (using small interfering RNA expression constructs) inhibits the chondrocyte cell cycle and proliferation in vitro and ex vivo. The silencing of PERK alone using siRNA against PERK (siPERK) led to arrest in the G1 phase, it decreased the number of cells in the S phase, and delayed progressoin to the G2-M phase. Co-transfection with siRNA against ATF (siATF4) led to a more profound inhibitory effect on cell cycle progression. Moreover, transfection with siPERK was associated with enhanced endoplasmic reticulum (ER) stress-induced apoptosis during bone morphogenetic protein 2 (BMP2)-induced chondrogenesis, and transfection with siATF4 exacerbated ER stress-related cell death. Data from flow cytometry (FCM), immunohistochemistry and TUNEL assays supported these findings in vitro and ex vivo. As shown by our results, the combined effect of the silencing of ATF4 and PERK led to the activation of an ER stress-specific caspase cascade in the cartilage tissue. On the whole, these findings reveal a new crucial combined effect of the silencing of PERK and ATF4 in modulating ER stress-mediated apoptosis during chondrocyte differentiation and proliferation.
Keywords: protein kinase R-like endoplasmic reticulum kinase, activating transcription factor 4, endoplasmic reticulum stress, apoptosis, chondrocyte differentiation
Introduction
The normal function of the endoplasmic reticulum (ER) is essential for numerous cellular processes and, ultimately, cell survival. Conditions that inhibit the protein folding capacity of the ER lead to the accumulation of misfolded or unfolded proteins in the ER lumen, generating a potentially toxic state referred to as ER stress. ER stress is attenuated through the activation of a complex adaptive cellular response, known as the unfolded protein response (UPR). Three transmembrane proteins, inositol-requiring enzyme 1 (IRE1), protein kinase R (PKR)-like ER kinase (PERK) and activating transcription factor (ATF)6, are responsible for detecting ER stress and the initiation of the UPR. Prolonged stress or failure to adapt to ER stress ultimately culminates in ER stress-induced apoptosis, and ER stress has been associated with various neurodegenerative, cardiovascular and orthopedic diseases (1–4). Additionally, ER stress has been demonstrated to play an important role in cellular differentiation during developmental processes. Accordingly, characterizing molecular mediators of the signaling switch between the protective and apoptotic responses to ER stress, and how these molecules interact to influence cell differentiation and proliferation, is an important endeavor which may reveal key aspects of developmental regulation and cellular pathologies.
Several studies have clearly demonstrated that physiological stressors influence cell differentiation and survival during musculoskeletal developmental and reparative processes, including chondrocyte differentiation, chondrogenesis and endrochondral ossification (5–7). Chondrocyte sensitivity to ER stress has been documented and a clear association between ER stress and several diseases affecting connective tissue is readily observable through murine genetic knockout studies and the analysis of diseased human tissues (8,9); however, the mechanisms through which ER stress specifically affects differentiation programs in chondrocytes remain poorly understood.
Of note, bone morphogenetic protein 2 (BMP2), a pre-eminent cytokine, plays critical roles in embryogenesis, cell growth, differentiation, bone development and the repair of bone fractures. It also activates UPR transducers, such as PERK, old astrocyte specifically-induced substance (OASIS) and ATF6 (10–12). Notably, PERK is a major transducer of the ER stress response and directly phosphorylates eukaryotic initiation factor 2α (eIF2α), which specifically promotes the translation of ATF4. PERK and ATF4 have been shown to play important roles in osteoblast differentiation and bone formation. Specifically, Saito et al. as well as others revealed that ER stress occured during BMP2-induced osteoblast differentiation and activated the PERK-eIF2α-ATF4 signaling pathway, followed by the promotion of gene expression essential for osteogenesis (13–15). In an effort to disentangle the dual association of PERK/ATF4 signaling with both pro-survival and pro-apoptotic responses during ER stress, Walter et al, and others, investigated the association between cell fate and the temporal activation of PERK/ATF4 in live cells and found that the shift from cell survival to apoptosis was determined by the timing of PERK/ATF4 signaling relative to that of IRE1/XBP1, another UPR signaling pathway (16,17).
However, whether PERK/ATF4 signaling participates in ER stress-mediated apoptosis during the course of chondrocyte differentiation, and the potential underlying mechanism(s), remain unknown. Thus, the current study aimed to better define the molecular mediators of cell survival during cartilage development with special regard to molecules associated with chondrocyte differentiation and ER stress-induced apoptosis. Specifically, the data presented herein elucidate the involvement of PERK and ATF4 in cell cycle progression and ER stress-mediated apoptosis during the course of chondrogenesis. Furthermore, the combined effect(s) of PERK and ATF4 upon the regulation of the cell cycle and apoptosis were investigated.
Materials and methods
Ethics statement
All animal experiments were designed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Science Foundation of China and conducted with the prior approval of the Chongqing Medical University Institutional Animal Care and Use Committee (permit nos. SYXK 2007-0001 and SCXK 2007-0002) and the Committee on the Ethics of Animal Experiments of Chongqing Medical University. Mice were housed under controlled temperatures in a 12 h light/dark cycle with easy access to food and water.
Adenoviruses
To generate PERK and ATF4 small interfering RNA (siRNA; siPERK and siATF4, respectively) expression constructs, siRNA corresponding to the coding sequence of the PERK and ATF4 genes (siPERK forward, 5′-ACCTCCAAGACCAACCACTTTTTT-3′ and reverse, 5′-AAAGTGGTTGGTCTTGGAGGTTTT-3′; and siATF4 forward, 5′-AGGAGCAAAACAAGACAGCATTTT-3′ and reverse, 5′-ATGCTGTCTTGTTTTGCTCCTTTT-3′) were cloned into the pSES-HUS vector (an adenoviral shuttle vector for siRNA expression, a gift from Professor Tangni, Chongqing Medical University) according to the manufacturer's instructions (18,28). All constructs were verified by nucleic acid sequencing; subsequent analysis was performed using BLAST software (National Institutes of Health, available at http://www.ncbi.nlm.nih.gov/blast/).
Cell culture
To examine the effect of knocking down PERK and ATF4 on chondrogenesis, ATDC5 chondrogenic cells (ATCC®; PCS-500-051™) and C3H10T1/2 embryonic firbro-blasts (a gift from Dr Chuanju Liu, New York University School of Medicine, New York, NY, USA) were infected with adenoviral vector containing siPERK or siATF4 or siPERK + siATF4 or a control RFP adenovirus before micromass culture. To examine the effect of the silencing of ATF4 and PERK on chondrogenesis, ATDC5 cells and C3H10T1/2 cells were infected with siATF4 (MOI=60) or siPERK (MOI=80) adenovirus or control RFP adenovirus prior to micromass culture. Uninfected cells were used as the negative controls (NC). Micromass culture was performed as described previously (19,20). The ATDC5 and C3H10T1/2 cells were briefly trypsinized and resuspended in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% FBS at a concentration of 106 cells/ml, and 6 drops (approximately 120 μl) of suspended cells were placed in a 60-mm tissue culture dish (Becton-Dickinson, San Diego, CA, USA). After 2 h of incubation at 37°C, 1 ml of DMEM containing 10% FBS and BMP2 (300 ng/ml) was added to the culture medium. The medium was changed every 2–3 days.
RNA extraction and reverse transcription (RT)-PCR
Total RNA was extracted from the cultured cells using the RNeasy Mini kit (Qiagen, Hilden, Germany) and reverse transcribed using the SuperScript pre-amplification system (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. The following sequence-specific primers were synthesized: 5′-AGCACTCAGATGGAGAGAGTCAG-3′ and 5′-GCTATGGGAGTTGTTGGACTGT-3′ for PERK; 5′-TGGCGTCCTCGGCCTTCAC-3′ and 5′-TTCCCTCCCTCCCTTTG ACG-3′ for ATF4. GAPDH was employed as an internal control using the following of oligonucleotides: 5′-ACC ACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTG TTGCTGTA-3′. The identity of each targeted PCR amplification product was confirmed by DNA sequence analysis of gel-purified bands (Qiagen).
Western blot analysis
Proteins in total cell extract from micromass cultures of BMP2-treated (300 ng/ml) ATDC5 or C3H10T1/2 cells were resolved on a 10% SDS-polyacrylamide gel and electroblotted onto nitrocellulose membranes. After blocking in 10% non-fat dry milk in Tris-buffered saline Tween-20 [10 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.5% Tween-20], the blots were incubated with either mouse monoclonal anti-PERK antibody (sc-377400; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and anti-C/EBP homologous protein (CHOP) antibody (ab11419; diluted 1:1,000; Abcam, Cambridge, UK) or rabbit anti-caspase-3 antibody (ab32351; Abcam) and anti-p-c-Jun N-terminal kinase (JNK) antibody (sc-293138; Santa Cruz Biotechnology, Inc.) for 1 h. After washing, the respective secondary antibody [horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin or HRP-conjugated anti-rabbit immunoglobulin; Sigma. St. Louis, MO, USA; A9044] was added, and the bound antibody was visualized using an enhanced chemiluminescence system (Amersham Biosciences, Uppsala, Sweden).
Culture of fetal mouse bone explants and immunohistochemistry
Metatarsals were isolated from 8 newborn C57BL/6J mice and cultured in DMEM (Gibco) containing 1% heat-inactivated fetal calf serum (Invitrogen) and 100 U/ml penicillin-strep tomycin in the presence of BMP2 (300 ng/ml) and siPERK, siATF4, or siPERK + siATF4 for 5 days, followed by histological examination. Affinity-purified anti-caspase-3 (ab32351; Abcam), caspase-12 (ab62484; Abcam), anti-CHOP (ab11419; Abcam) and anti-p-JNK (sc-293138; Santa Cruz Biotechnology, Inc.) were diluted at 1:100 and sections were incubated at room temperature for 2 h. For detection, biotinylated secondary antibody (sc-2364) and HRP-streptavidin complex (sc-2363) (Santa Cruz Biotechnology, Inc.) were used. A total of 0.5 mg/ml 3,3′-diami-nobenzidine (DAB) in 50 mM Tris-HCl substrate (Sigma) was used for visualization, and the sections were counterstained with Mayer's hematoxylin (H9627; Sigma).
TUNEL assay
Metatarsals from 5 newborn C57BL/6J mice, as well as micromass cultures of C3H10T1/2 and ATDC5 cells, were cultured for 5 days in the presence of conditioned medium containing BMP2 (300 ng/ml) and siPERK, siATF4, or siATF4 + siPERK followed by the detection of apoptosis in accordance with the manufacturer's recommended protocol using the DeadEnd™ Fluorometric TUNEL System (Promega Corp., Madison, WI, USA). Localized green fluorescence of apoptotic FITC-labeled TUNEL-positive cells was imaged using a fluorescence microscope (S/N:2109; Kramer Scientific Corp., Amesbury, MA, USA).
Apoptosis analysis by flow cytometry (FCM)
At 48 h following infection with siPERK, siATF4, or siPERK + siATF4, ATDC5 and C3H10T1/2 cells in micromass culture were then cultivated in BMP2 (300 ng/ml) for 1, 3 and 5 days. Each culture media of BMP2-treated ATDC5 and C3H10T1/2 cells were then collected for FCM analysis. Briefly, following incubation with RNase (1 mg/ml; Qiagen) in the dark at 37°C for 1 h, the cells were stained with propidium iodide (30 mg/ml; Sigma) and analyzed using a flow cytometer (FACSCalibur; Becton-Dickinson) to determine cell cycle distribution and detect cellular apoptotic rate. The experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 10.0.1 software for Windows. Data are expressed as the means ± SD from at least 3 independent experiments. The Student's t-test was used to determine whether two sets of data were significantly different from each other. Data for multiple variable comparisons were analyzed by one-way analysis of variance (ANOVA). A value of P<0.05 was deemed to indicate a statistically significant difference.
Results
Measuremnt of ATF4 and PERK expression following transfection with specific siRNA constructs
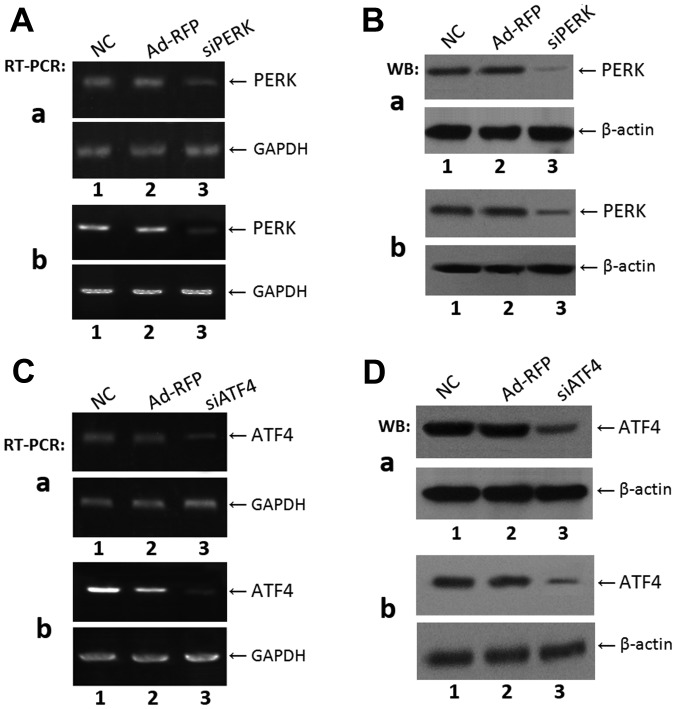
siPERK and siATF4 adenoviral vectors were constructed and identified using endonuclease digestion and DNA sequencing (data not shown). The ATDC5 and C3H10T1/2 cells infected with siPERK, siATF4, or siPERK + siATF4 were examined by RT-PCR and western blot analysis. The mRNA level of PERK markedly decreased in the ATDC5 and C3H10T1/2 cells transfected with siPERK, as compared with the untransfected controls (Fig. 1A). The protein level was also significantly decreased in the siPERK-infected cells, as compared with the control cells (Fig. 1B). Furthermore, as shown in Fig. 1C and D, the mRNA expression of ATF4 decreased compared to the controls, in the siATF4-infected ATDC5 and C3H10T1/2 cells. The ATF4 protein level was also significantly decreased in the siATF4-infected ATDC5 and C3H10T1/2 cells compared with the respective control cells. These results confirm the successful construction of the adenoviral vectors and the silencing of PERK and ATF4.
Figure 1.
Expression of PERK and ATF4 in ATDC5 and C3H10T1/2 cells after infection with siPERK or siATF4. (A) Analysis of PERK mRNA level with RT-PCR in (a) ATDC5 and (b) C3H10T1/2 cells. Lane 1, NC; lane 2, adenovirus control Ad-RFP; lane 3, siPERK. (B) Determination of PERK protein expression level by western blot analysis after infection with siPERK in (a) ATDC5 and (b) C3H10T1/2 cells. Lane 1, NC; lane 2, Ad-RFP control group; lane 3, siPERK group. Proteins were separated by 10% SDS-PAGE and analyzed with anti-PERK antibody. GAPDH and β-actin were used as internal controls in RT-PCR and western blotting, respectively. The levels of PERK mRNA and proteins were markedly increased after infection with siPERK as compared with control groups. (C) Analysis of ATF4 mRNA level by RT-PCR in (a) ATDC5 and (b) C3H10T1/2 cells (b). Lane 1, NC; lane 2, adenovirus control Ad-RFP; lane 3, siATF4. (D) Determination of ATF4 protein expression level after infection with siATF4 in (a) ATDC5 and (b) C3H10T1/2 cells. Lane 1, NC; lane 2, adenovirus control group Ad-RFP; lane 3, siATF4 group. Proteins were separated by 10% SDS-PAGE and analyzed with anti-ATF4 antibody. GAPDH and β-actin were used as internal controls in RT-PCR and western blot analysis, respectively. The protein and mRNA levels of ATF4 were markedly reduced after infection with siATF4 as compared with the control groups. PERK, PKR-like ER kinase; ATF4, activating transcription factor 4.
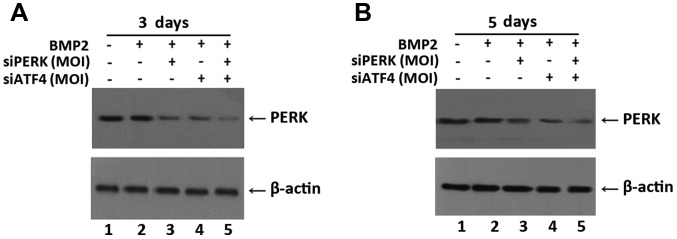
Silencing of ATF4 by transfection with siATF4 decreases the expression of PERK in chondrogenesis
To further investigate the function of PERK in chondrogenesis, we examined the expression of PERK in a BMP2-induced micromass culture of ATDC5 cells by western blot analysis. As shown in Fig. 2A, the silencing of PERK by transfection with siPERK markedly decreased the expression of PERK in the BMP2-stimulated ATDC5 cells after 3 days of culture. Furthermore, concurrent silencing of the expression of PERK and ATF4 in the BMP2-stimulated ATDC5 cells further decreased PERK expression compared to the cells in wich only PERK was silenced. These results remained consistent after 5 days of the BMP2-induced micromass culture of ATDC5 cells as assessed by western blot analysis. As shown in Fig. 2B, in the cells treated with BMP2 + siATF4 + siPERK PERK expression was markedly decreased compared with the BMP2 + siPERK and BMP2 + siATF4 groups. These data clearly indicate that the silencing of ATF4 by transfection with siATF4 regulates endogenous PERK protein expression, and further reduces the expression of PERK inhibited by siPERK. Micromass cultures of these cells were incubated in the presence of 300 ng/ml BMP2 for the induction of chondrogenesis.
Figure 2.
siATF4 decreases the expression of PERK in siPERK-infected ATDC5 cells during chondrogenesis. (A) Determination of PERK protein expression level after infection with siPERK, siATF4, and siATF4 + siPERK in BMP2-stimulated cells at 3 days by western blot analysis. Lane 1, NC; lane 2, BMP2 group; lane 3, BMP2 + siPERK group; lane 4, BMP2 + siATF4 group; lane 5, BMP2 + siPERK + siATF4 group. Proteins were separated by 10% SDS-PAGE and analyzed with anti-PERK antibody. β-actin was used as internal control in western blot analysis. The protein levels of PERK were markedly decreased after infection with BMP2 + siPERK + siATF4 compared with other groups. (B) Determination of PERK protein expression level after infection with siPERK, siATF4, and siATF4 + siPERK after 5 days of BMP2-induced culture by western blot analysis. Lane 1, NC; lane 2, BMP2 group; lane 3, BMP2 + siPERK group; lane 4, BMP2 + siATF4 group; lane 5, BMP2 + siPERK + siATF4 group. Proteins were separated by 10% SDS-PAGE and analyzed with anti-PERK antibody. β-actin was used as internal control in western blot analysis. The protein level of PERK was obviously decreased after infection with BMP2 + siPERK + siATF4 compared with the other groups. PERK, PKR-like ER kinase; BMP2, bone morphogenetic protein 2.
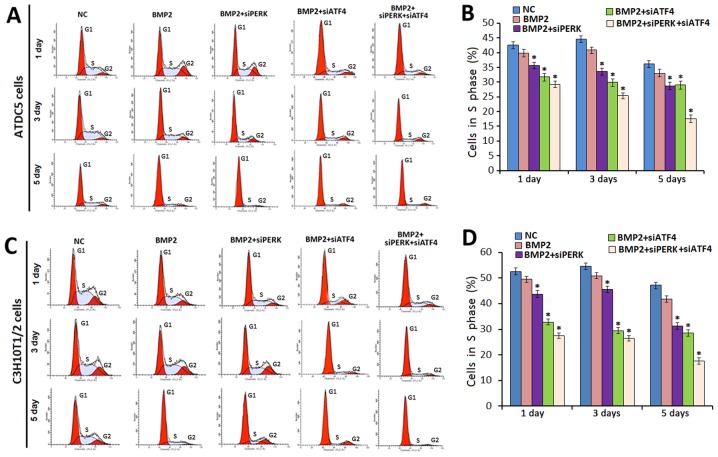
Combined effect of the silencing of ATF4 and PERK on cell growth in chondrocyte differentiation
To examine whether the knockdown of PERK and/or ATF4 can influence the cell cycle profile during chondrogenesis, FCM analysis was used to determine the cell cycle distributions of ATDC5 and C3H10T1/2 cells during BMP2-induced chondrogenesis in the presence of siPERK, siATF4 or siATF4 + siPERK.
At the time points of 1, 3, and 5 days, the proportion of ATDC5 cells in the S phase following treatment with BMP2 + siPERK was decreased compared to that of the ATDC5 cells cultured under the influence of BMP2 alone (Fig. 3A and B). In the ATDC5 cells treated with BMP2 + siPERK, the cell number in the S phase was 36.03, 34.21 and 28.17% on days 1, 3, and 5, respectively; for the ATDC5 cells treated with BMP2 + siATF4, 32.17, 30.78, and 29.25% of the cell population was in the S phase on days 1, 3, and 5, respectively. The decreased percentage of cells in the S phase was even more significant in the ATDC5 cells treated with BMP2 + siATF4 + siPERK, with S phase cells accounting for only 29.78, 26.52 and 17.82% of the cell population at 1, 3, and 5 days, respectively.
Figure 3.
Analysis of cellular proliferation by FCM. (A and C) FCM images with propidium iodide staining and analysis on cell cycle distribution. Micromass culture of (A) ATDC5 and (C) C3H10T1/2 cells after treatment with NC/BMP2 (300 ng/ml)/BMP2 + siPERK/BMP2 + siATF4/BMP2 + siPERK + siATF4. FCM analysis showed that the percentage of the BMP2 + siPERK + siATF4 ATDC5 cells in he S phase was decreased significantly comapred to that of the cells in the BMP2 + siPERK, BMP2 + siATF4 and BMP2 control groups during BMP2-induced chondrogenesis at 1,3, and 5 days. The results for the C3H10T1/2 cells were similar. Experiments were repeated 3 times, and samples were analyzed by the Student's t-test and statistical significance with P<0.05. Representative images are shown. (B and D) FCM analysis showed that the percentages of ATDC5 and C3H10T1/2 cells in the BMP2 + siPERK + siATF4 groups in the S phase were decreased significantly compared with those of the cells in the BMP2 + siPERK, BMP2 + siATF4 and BMP2 control groups. *P<0.05 compared with the control. FCM, flow cytometry; BMP2, bone morphogenetic protein 2.
Similarly, in the C3H10T1/2 cells treated with BMP2 + siPERK, the percentage of cells in the S phase was 42.35, 44.07, 31.21%; and 33.05, 29.01, 27.65% in C3H10T1/2 cells treated by BMP2 + siATF4 (1, 3, and 5 days, respectively). In addition, 28.91, 27.45 and 18.37% of C3H10T1/2 cells treated with BMP2 + siATF4 + siPERK were recorded to be in the S phase on 1, 3, and 5 days, respectively (Fig. 3C and D).
The difference between the S phase cell distribution data for each treatment group and that of the corresponding control groups reached statistical significance (P<0.05). These data indicate that the knockdown of PERK inhibits cell cycle distribution during chondrogenesis, and that the silencing of ATF4 enhances the inhibitory effect of the silencing of PERK on cell growth during chondrocyte differentiation.
The FCM data also revealed that in the ATDC5 cells treated with BMP2 + siATF4 + siPERK, the percentage of cells in the G2 phase was 18.82±0.91, 9.31±1.02, 6.07±0.85% on culture days 1, 3, and 5 days, respectively. In the C3H10T1/2 cells treated with BMP2 + siATF4 + siPERK, 17.75±0.92, 8.51±0.92, and 5.87±0.94% of cells were in the G2 phase at 1, 3, and 5 days of culture, respectively (Fig. 3, P<0.05). Collectively, these results indicate that the silencing of PERK affects cell cycle distribution by reducing the number of cells in the S phase cells during chondrogenesis. Specifically,the knockdown of PERK inhibited cell proliferation during chondrocyte development with arrest in the G1 phase, a decrease in the number of cells in the S phase and the delay of the progression to the G2-M phase. Furthermore, the silencing of ATF4 enhanced the inhibitory effect of the silencing of PERK on cell cycle progression during chondrocyte differentiation.
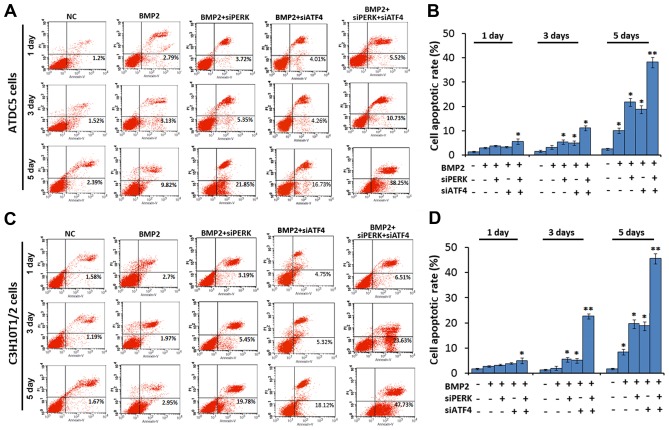
Combined effect of the silencing of ATF4 and PERK on ER stress-mediated apoptosis
We then sought to determine whether the silencing of PERK (using siPERK), ATF4 (using siATF4) or both (using siATF4 + siPERK) affects cell apoptosis. As shown in Fig. 4, the cell apoptotic rate was markedly increased after micromass culture of the ATDC5 cells for 1, 3 and 5 days following treatment with BMP2 + siATF4 + siPERK, as compared with the BMP2 + siPERK, BMP2 + siATF4 and BMP2 treatment groups. The cell apoptotic rate was 3.72, 5.35 and 21.85% in the BMP2 + siPERK-treated ATDC5 cells, and 4.01, 4.26 and 16.73% in the BMP2 + siATF4-treated ATDC5 cells on 1, 3 and 5 days, which clearly reflects a larger population of apoptotic cells as compared with the cells treated with BMP2 alone. In the BMP2 + siATF4 + siPERK-treated ATDC5 cells, the cell apoptotic rate was markedly increased to 5.52, 10.73 and 38.25% on days 1, 3 and 5, respectively. The differences between each treatment group and the BMP2 control group reached statistical significance (P<0.05 and P<0.01, Fig. 4A and B).
Figure 4.
Analysis of apoptosis by FCM. (A and C) FCM analysis with Annexin V-PI staining was performed to evaluate the percentage of apoptotic cells in the BMP2 (300 ng/ml)-stimulated (A) ATDC5 and (C) C3H10T1/2 cells on culture days 1, 3 and 5. The percentage of apoptotic ATDC5 and C3H10T1/2 cells in the BMP2 + siPERK + siATF4 groups was significantly increased compared with that of the cells in the BMP2 + siPERK, BMP2 + siATF4 cell groups, and the controls. (B and D) Analysis of results of cell apoptosis. Data are presented as the means ± SD for relative apoptosis normalized to control cells for 3 independent experiments. Columns represent the means of 4 separate experiments; bars represent SD. *P<0.05 as determined by the Student's t-test, vs. BMP2 and BMP2 + siPERK + siATF4 group; **P<0.01, vs. BMP2 + siPERK and BMP2 + siPERK + siATF4 group; BMP2 + siATF4 and BMP2 + siPERK + siATF4 group. Representative images from FCM analysis are shown. FCM, flow cytometry; BMP2, bone morphogenetic protein 2.
Additionally, in the micromass culture of C3H10T1/2 cells, the cell apoptotic rate was also increased in the BMP2 + siPERK group (3.19, 5.45 and 19.78%, days 1, 3 and 5) and BMP2 + siATF4 group (4.75, 5.32 and 18.12%, days 1, 3 and 5) compared to the BMP2 group (2.7, 1.97 and 2.95%, days 1, 3 and 5). In the C3H10T1/2 cells transfected with both siPERK and siATF4, the apoptotic rate was 6.51, 23.63 and 47.73% on 1, 3 and 5 days, respectively. The differences between each treatment group and the BMP2 control group reached statistical significance (P<0.05 and P<0.01, Fig. 4C and D).
Taken together, these data demonstrate that the knockdown of PERK using siPERK or ATF4 using siAT4 enhances ER stress-mediated apoptosis in BMP2-induced chondrocyte differentiation. Further, the combined application of siPERK and siATF4 further promoted ER stress-mediated apoptosis in chondrocyte differentiation induced by BMP2, generating an additive 'push' toward apoptosis.
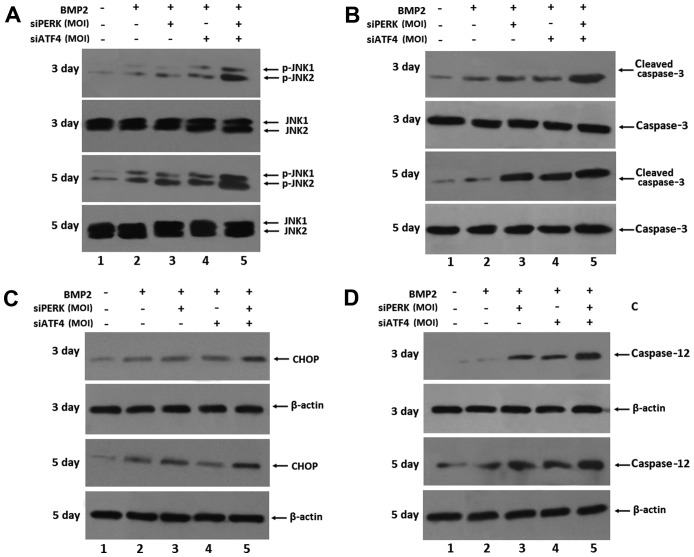
To confirm the influence of ER stress-mediated apoptosis by transfection with siATF4 and siPERK in BMP2-stimulated ATDC5 cells, the expression of ER stress-mediated apoptotic molecules, including CHOP, caspase-3, caspase-12 and p-JNK, was detected by western blot analysis in the ATDC5 cells stimulated with BMP2 for 3 and 5 days. The expression of CHOP, caspase-3, caspase-12 and p-JNK was markedly increased in the ATDC5 cells following infection with siPERK, siATF4 and siATF4 + siPERK, and BMP2 treatment over 3 and 5 days. As shown in Fig. 5, both siPERK and siATF4 induced a marked increase in the expression levels of p-JNK, active (cleaved) caspase-3, caspase-12 and CHOP in the ATDC5 cells transfected with siPERK. Furthermore, the expression levels of p-JNK, cleaved caspase-3, caspase-12 and CHOP were markedly increased following 3 and 5 days of BMP2 induction in the siATF4 + siPERK-infected ATDC5 cells. These results indicated that transfection with siPERK, siATF4 and siPERK + siATF4 increased the expression of ER stress-mediated apoptosis signaling pathway molecules during chondrocyte differentiation. The individual effects of the silencing of PERK and ATF4 exerted a more robust, additive combined effect as observed with the implementation of the siPERK + siATF4 treatment condition.
Figure 5.
Effects of transfection with siPERK + siATF4, siPERK and siATF4 on the expression of ER stress-mediated apoptosis in BMP2-stimulated ATDC5 cells for 3 and 5 days. (A) Expression of p-JNK in the course of chondrogenesis in a micromass culture of BMP2-stimulated ATDC5 cells for 3 and 5 days. Whole cell lysates were prepared from ATDC5 cells and treated with 300 ng/ml BMP2 for 3 and 5 days. The lysates were resolved by SDS-PAGE and then immunoblotted with antibodies against p-JNK and total JNK. (B) Expression of cleaved caspase-3 in the course of chondrogenesis in a micromass culture of BMP2-stimulated ATDC5 cells for 3 and 5 days. Whole cell lysates were prepared from ATDC5 cells and treated with 300 ng/ml BMP2 for 3 and 5 days. The lysates were resolved by SDS-PAGE and then immunoblotted with antibodies against cleaved caspase-3 and caspase-3. (C) Expression of CHOP in the course of chondrogenesis in a micromass culture of BMP2-stimulated ATDC5 cells for 3 and 5 days. Whole cell lysates were prepared from ATDC5 cells and treated with 300 ng/ml BMP2 for 3 and 5 days. The lysates were resolved by SDS-PAGE and then immunoblotted with antibodies against CHOP and β-actin. (D) Expression of caspase-12 in the course of chondrogenesis in a micromass culture of BMP2-stimulated ATDC5 cells for 3 and 5 days. Whole cell lysates were prepared from ATDC5 cells and treated with 300 ng/ml BMP2 for 3 and 5 days. The lysates were resolved by SDS-PAGE and then immunoblotted with antibodies against caspase-12 and β-actin. BMP2, bone morphogenetic protein 2; JNK, c-Jun N-terminal kinase; CHOP, C/EBP homologous protein.
The silencing of both ATF4 and PERK (siATF4 + siPERK) increases ER stress-mediated apoptosis in vitro
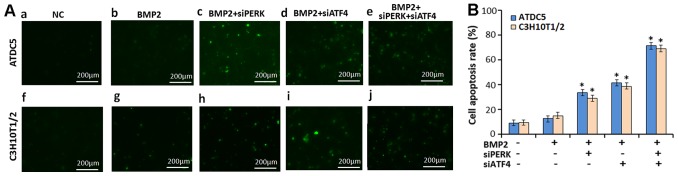
The high-density culture system was then incubated in the absence (CTR) or presence of 300 ng/ml BMP2, BMP2 + siPERK, BMP2 + siATF4 and BMP2 + siATF4 + siPERK for 3 days, at which point, a TUNEL assay was performed to examine the effects of siPERK and siATF4 + siPERK on apoptosis during chondrogenesis. As shown in Fig. 6, during BMP2-induced chondrocyte differentiation, the number of TUNEL-positive cells increased significantly in the ATDC5 cells transfected with BMP2 + siPERK (34.36%), BMP2 + siATF4 (42.85%) compared with the ATDC5 cells treated with BMP2 only (15.53%), and the number of TUNEL-positive cells was further enhanced in the ATDC5 cells treated with BMP2 + siA TF4 + siPERK (69.71%).
Figure 6.
Transfection with siATF4 + siPERK increases ER stress-mediated apoptosis in micromass culture of ATDC5 and C3H10T1/2 cells during BMP2-induced chondrogenesis. (A) Following treatment with 300 ng/ml BMP2, BMP2 + siPERK, BMP2 + siATF4 or BMP2 + siPERK + siATF4 in micromass culture of (a–e) ATDC5 and (f–j) C3H10T1/2 cells, the cells were then analyzed for apoptosis by TUNEL staining. Representative photographs of TUNEL staining in cells. The FITC-labeled TUNEL-positive cells were imaged under a fluorescence microscope (×200 magnification). The cells with green fluorescence were recognized as apoptotic cells, and the scale bars represent 200 μm. Representative images from TUNEL analysis are shown. (B) Analysis of results of cell apoptosis. Data are the means ± SD for relative apoptosis normalized to control cells for 3 independent experiments. Columns are the means of 3 separate experiments; bars represent SD. *P<0.05 as determined by Student's t-test, vs. BMP2 and BMP2 + siPERK group; BMP2 and BMP2 + siATF4 group; BMP2 + siATF4/BMP2 + siPERK and BMP2 + siPERK + siATF4 group. Representative images from TUNEL analysis are shown. BMP2, bone morphogenetic protein 2.
In addition, in the C3H10T1/2 cells treated with BMP2 + siPERK, and those treated with BMP2 + siATF4, the number of TUNEL-positive cells increased (32.83 and 39.36%) compared with that of the C3H10T1/2 BMP2-treated cells (17.68%). The number of TUNEL-positive C3H10T1/2 cells treated with BMP2 + siATF4 + siPERK (66.52%) was also significantly greater than that recorded from either the C3H10T1/2 BMP2 + siPERK-treated cells or BMP2 + siATF4-treated cells. TUNEL assay was repeated in triplicate. The differences between the BMP2 + siATF4 + siPERK, BMP2 + siPERK, BMP2 + siATF4 and BMP2 groups reached statistical significance (P<0.05, Fig. 6). It should be noted that the silencing of PERK and ATF4 activated ER stress-mediated apoptosis during chondrocyte differentiation induced by BMP2; the silencing of both ATF4 and PERK (siATF4 + siPERK) enhanceed ER stress-mediated apoptosis to a level exceeding that induced by the silencing of ATF4 or PERK alone.
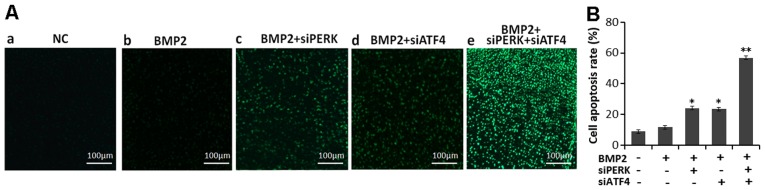
In order to verify whether the silecing of PERK (using siPERK), ATF4 (using siATF4) or both (siATF4 + siPERK) affects growth plate chondrocytes in developing tissue, TUNEL assay was undertaken to determine the effect of siPERK, siATF4 and siATF4 + siPERK on apoptosis in cartilage tissue (Fig. 7). The result show that the TUNEL-positive cells in the BMP2 + siATF4 + siPERK group were significantly increased compared with the BMP2 + siPERK, BMP2 + siATF4 and the BMP2 group. These results further demonstrate that a cumulative effect of siATF4 and siPERK in pushing chondrocytes toward an apoptotic cell fate.
Figure 7.
TUNEL assay in the chondrocytes in growth plate ex vivo. (A) Representative images of TUNEL staining in cells. The newborn mouse metatarsals were explanted and cultured in the presence of conditioned medium of (b) BMP2 (300 ng/ml), (c) BMP2 + siPERK, (d) BMP2 + siATF4 or (e) BMP2 + siPERK + siATF4 for 5 days and were then analyzed for apoptosis by TUNEL-staining assay, (a) NC is the control. The FITC-labeled TUNEL-positive cells were imaged under a fluorescence microscope (×100 magnification). The cells with green fluorescence were recognized as apoptotic cells, and the scale bars represent 100 μm. (B) Analysis of results of cell apoptosis. Data are the means ± SD for relative apoptosis normalized to control cells for 3 independent experiments. Columns are the means of 3 separate experiments; bars represent SD. *P<0.05 as determined by Student's t-test, vs. BMP2 and BMP2 + siPERK group; BMP2 and BMP2 + siATF4 group; **P<0.01, vs. BMP2 and BMP2 + siPERK + siATF4 group. BMP2, bone morphogenetic protein 2.
Silencing of ATF4 and PERK induces ER stress-mediated caspase activation in chondrocyte tissue
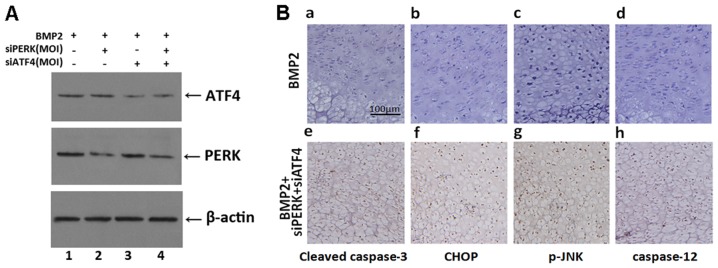
To further understand the molecular events of ER stress-mediated apoptosis induced by the silencing of ATF4 and PERK (siATF4 + siPERK) in chondrogenesis, the effect of transfection with siATF4 + siPERK on endochondral bone formation was examined by implementing cultures of metatarsals isolated from newborn mice as an ex vivo model of bone formation. Firstly, the metatarsals were cultured for 5 days in the presence of conditioned medium containing 300 ng/ml BMP2 (control), BMP2 + siPERK, BMP2 + siATF4 or BMP2 + siATF4 + siPERK adenovirus. Western blot analysis was then used to examine the expression of ATF4 and PERK in the metatarsal culture extracts. The protein level of ATF4 was markedly decreased in the siATF4 and siPERK + siATF4-infected culture extracts, as compared with the protein level of ATF4 in the BMP2 and BMP2 + siPERK-treated culture extracts. Likewise, the protein level of PERK was markedly decreased in the siPERK and siPERK + siATF4-infected culture extracts, as compared to the BMP2 and BMP2 + siATF4 treatment group (Fig. 8A).
Figure 8.
Expression of cleaved caspase-3, CHOP, p-JNK and caspase-12 in the growth plate chondrocytes in vivo. Metatarsals were explanted from newborn mouse embryos and cultured in the presence of conditioned medium of BMP2 (300 ng/ml), BMP2 + siATF4 + siPERK for 5 days. (A) Western blot analysis was used to detect the expression of ATF4 and PERK in metatarsals cultured in the presence of conditioned medium of BMP2 (300 ng/ml), BMP2 + siPERK, BMP2 + siATF4 or BMP2 + siPERK + siATF4 for 5 days. (B, a and e) Immunohistochemistry staining was observed in low-power microphotograph of a section stained with anti-active caspase-3 monoclonal antibody (brown) and counterstained with Mayer's hematoxylin (blue). (b and f) Immunohistochemistry staining was observed in low-power microphotograph of a section stained with anti-CHOP monoclonal antibody (brown) and counterstained with Mayer's hematoxylin (blue). (c and g) Immunohistochemistry staining was observed in low-power microphotograph of a section stained with anti-p-JNK monoclonal antibody (brown) and counterstained with Mayer's hematoxylin (blue). (d and h) Immunohistochemistry staining was observed in low-power microphotograph of a section stained with anti-caspase-12 monoclonal antibody (brown) and counterstained with Mayer's hematoxylin (blue) and the scale bars represent 100 μm. CHOP, C/EBP homologous protein; BMP2, bone morphogenetic protein 2; ATF4, activating transcription factor 4; PERK, PKR-like ER kinase.
We then detected the expression of ER stress-specific caspases. At the time of explantation, these explants consisted of undifferentiated cartilage. Over a 5-day culture period, these explants underwent all sequential stages of endochondral bone formation. As shown in Fig. 8B, treatment with siATF4 + siPERK increased the expression of apoptosis-related proteins, such as cleaved caspase-3, CHOP, p-JNK and caspase-12.
These results demonstrated the activation of caspase-3, p-JNK, CHOP and caspase-12 by ER stress during chondro-genesis and that the silecing of ATF4 and PERK increased the expression of ER stress-mediated apoptosis signaling pathway molecules. Taken together, these data demonstrated that the combined silencing of ATF4 and PERK enhanced ER stress-mediated apoptosis in BMP2-induced chondrogenesis.
Discussion
In eukaryotic cells, signaling pathways relay information between the ER, cytosol and nuclei to restrict the accumulation of unfolded proteins in the ER. A number of studies have shown that factors influencing cell fate and/or differentiation are activated during ER stress. In mammalian cells, the UPR plays a fundamental role in maintaining cellular homeostasis and is therefore at the center of many normal physiological responses and pathologies (21–24).
Cells respond to ER stress via ER stress sensors, leading to the UPR. PERK is a major transducer of the ER stress response and directly phosphorylates eIF2α, resulting in translational attenuation (16,25,26). Whether and how PERK/ATF4 participates in ER stress-mediated apoptosis in the process of chondrocyte differentiation, and the mechanisms of how ER stress-mediated apoptosis is regulated in chondrogenesis remain unknown.
Our current study aimed to address the combined effect of the silencing of PERK and ATF4 on ER stress-mediated apoptosis during the process of chondrogenesis, as well as to elucidate the molecular mechanisms involved. To define the influence of these molecules, we first adenoviral vectors carrying siPERK and siATF4, and infected the ATDC5 and C3H10T1/2 cells. Protein analysis of whole cell extracts validated our approach, as the expression of PERK and ATF4 was markedly decreased in each of the cells expressing the relevant adenoviral vectors (Fig. 1). Furthermore, we demonstrated that the silencing of ATF4 was able to regulate endogenous PERK gene expression, evidenced by the further reduction in PERK expression in the cells co-transfected with siATF4 and siPERK, as compared to the cells transfected with siPERK alone (Fig. 2).
We previously reported that BMP2 mediates mild ER stress during chondrogenesis and activates the IRE1α-XBP1 pathway; X-box binding protein 1 spliced (XBP1s) in turn enhances chondrocyte hypertrophy by functioning as a co-factor of RUNX2. We also previously found that BMP2 activates UPR-signaling molecules in chondrogenesis, such as XBP1s, BiP and IRE1α (27,28). Herein, we expanded upon our previous findings by defining the role of PERK/ATF4 in ER stress-mediated apoptosis during chondrocyte differentiation. Our current data indicate that PERK/ATF4 influences cell cycle distribution in chondrogenesis. Firstly, the application of siPERK and siATF4 inhibited cell proliferation in chondrocyte development with G1 phase arrest, a reduction in the number of cells in the S phase and the delay of G2-M phase progression. The joint application of siATF4 and siPERK resulted in the enhanced disruption of cell cycle distribution (Fig. 3). FCM analysis illustrated that siPERK and siATF4 enhanced ER stress-mediated apoptosis in chondrogenesis induced by BMP2, and siATF4 also enhanced the apoptotic effect of siPERK (Fig. 4).
ER stress-induced cell death is a new, exciting apoptotic pathway, the full impact of which, particularly in development and the pathology of disease, remains undetermined (29,30). It is known that caspases, a family of cysteine proteases including caspase-3, -9, and -12, act as a common death effect or molecules in various forms of apoptosis. Caspase-12 is an ER-associated proximal effector in the caspase activation cascade, and cells lacking this enzyme are partially resistant to inducers of ER stress (31–33). Three pathways have been identified as being involved in ER stress-mediated apoptosis: the caspase-12/caspase-4 pathway, the CHOP pathway and the IRE1-JNK pathway. Caspase-12 and -4 have been proposed as caspases that initiate ER stress-induced cell death with caspase-12 reported to directly cleave pro-caspase-9 and induce apoptosis (34–37). CHOP induces ER stress-induced cell death, at least in part, by suppressing the expression of Bcl-2 and inducing Bim expression. It has been reported that IRE1a also participates in ER stress-induced cell death by activating JNK. These findings support the notion that ER stress leads to several redundant pathways for caspase activation (38–40).
In order to gauge the activation of ER-stress-mediated apoptotic pathways, we detected the expression of phosphorylated JNK, cleaved caspase-3, CHOP and caspase-12 following treatment with BMP2/BMP2 + siPERK/BMP2 + siATF4/BMP2 + siPERK + siATF4. The expression levels of phosphorylated JNK, cleaved caspase-3, CHOP and caspase-12 were increased in the cells treated with BMP2 + siPERK + siATF4 as compared with those treated with BMP2 + siPERK or BMP2 + siATF4 or BMP2 alone (Fig. 5). Additionally, the expression of ER stress-mediated apoptosis signaling pathway-associated molecules was also increased in the BMP2 + siPERK group and BMP2 + siATF4 group as compared to the BMP2 treatment control group. Accordingly, we demonstrated that transfection with siPERK and siATF4 increased the expression of ER stress-mediated apoptotic signaling pathway molecules during chondrogenesis and that co-transfection with siATF4 enhanced the upregulated expression of apoptotic molecules induced upon treatment with siPERK.
Furthermore, the results of TUNEL assay and immunohistochemistry revealed that the BMP2 + siATF4 + siPERK group featured many more apoptotic cells as compared with the BMP2 + siPERK group, BMP2 + siATF4 group and the BMP2 group, demonstrating that the silencing of PERK and ATF4 increased the expression of ER stress-mediated apoptosis signaling pathway molecules during chondrogenesis (Figs. 6–8).
In a word, our data indicate that the silencing of PERK and ATF4 enhance ER stress-mediated apoptosis during chondrogenesis and that the joint silencing of ATF4 and PERK leads to a more profound promotion of apoptotic signaling that is observed following the silencing of either PERK or ATF4 alone.
In conclusion, this study provides novel insight into the role of PERK and ATF4 in regulating ER stress-mediated apoptosis during chondrocyte differentiation. Our study supports the notion that the knockdown of PERK, a key regulator of the mammalian UPR, decreases the growth of chondrocytes and induces ER stress-mediated apoptosis during chondrogenesis. The knockdown of ATF4 using siATF4 similarly resulted in the decreased growth of chondrocytes and greater ER stress-mediated apoptosis during chondrogenesis. The simultaneous knockdown of PERK and ATF4 augmented ER stress-mediated apoptosis, surpassing the levels observed under the knockdown conditions of ATF4 or PERK alone, during chondrocyte differentiation. Continued research in this field is necessary to clarify the complexities of this cell-death pathway. New insight into the mechanistic basis of stress responses will open new perspectives for the development of molecular target-based treatment approaches, and may thus have great potential for use in the treatment of cartilage disorders and arthritic conditions.
Acknowledgments
The authors would like to thank Aubryanna Hettinghouse (Department of Orthopaedic Surgery and Cell Biology, New York University School of Medicine) for critically reading the manuscript. This study was supported by the National Natural Science Foundation of China (81371928 and 81171697); New Century Excellent Talent Support Project of Education Ministry of China (NCET-12-1090).
Abbreviations
- BMP2
bone morphogenetic protein 2
- ATF6
activating transcription factor 6
- ERS
ER stress
- UPR
unfolded protein response
- PERK
protein kinase R-like endoplasmic reticulum kinase
- IRE1α
inositol-re quiring enzyme 1α
- XBP1s
X-box binding protein 1 spliced
References
- 1.Pluquet O, Pourtier A, Abbadie C. The unfolded protein response and cellular senescence. A review in the theme: Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol Cell Physiol. 2015;308:C415–C425. doi: 10.1152/ajpcell.00334.2014. [DOI] [PubMed] [Google Scholar]
- 2.Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol. 2016;16:469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karali E, Bellou S, Stellas D, Klinakis A, Murphy C, Fotsis T. VEGF signals through ATF6 and PERK to promote endothelial cell survival and angiogenesis in the absence of ER stress. Mol Cell. 2014;54:559–572. doi: 10.1016/j.molcel.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, Sekiya H, Tsumagari K, Ochiai K, Yoshinaga K, et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol. 2009;11:1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- 5.Horiuchi K, Tohmonda T, Morioka H. The unfolded protein response in skeletal development and homeostasis. Cell Mol Life Sci. 2016;73:2851–2869. doi: 10.1007/s00018-016-2178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuscik MJ, Hilton MJ, Zhang X, Chen D, O'Keefe RJ. Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest. 2008;118:429–438. doi: 10.1172/JCI34174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito A, Imaizumi K. Endoplasmic reticulum stress response in osteogenesis. Clin Calcium. 2013;23:1569–1575. In Japanese. [PubMed] [Google Scholar]
- 8.Saito A, Hino S, Murakami T, Kanemoto S, Kondo S, Saitoh M, Nishimura R, Yoneda T, Furuichi T, Ikegawa S, et al. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat Cell Biol. 2009;11:1197–1204. doi: 10.1038/ncb1962. [DOI] [PubMed] [Google Scholar]
- 9.Rajpar MH, McDermott B, Kung L, Eardley R, Knowles L, Heeran M, Thornton DJ, Wilson R, Bateman JF, Poulsom R, et al. Targeted induction of endoplasmic reticulum stress induces cartilage pathology. PLoS Genet. 2009;5:e1000691. doi: 10.1371/journal.pgen.1000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009;20:475–480. doi: 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Canalis E, Economides AN, Gazzerro E. Bone morpho-genetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 12.Jang WG, Kim EJ, Kim DK, Ryoo HM, Lee KB, Kim SH, Choi HS, Koh JT. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J Biol Chem. 2012;287:905–915. doi: 10.1074/jbc.M111.253187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, Cavener DR, Imaizumi K. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem. 2011;286:4809–4818. doi: 10.1074/jbc.M110.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu CY, Schröder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem. 2000;275:24881–24885. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- 15.Tsai SF, Tao M, Ho LI, Chiou TW, Lin SZ, Su HL, Harn HJ. Isochaihulactone-induced DDIT3 causes ER stress-PERK independent apoptosis in glioblastoma multiforme cells. Oncotarget. 2016 doi: 10.18632/oncotarget.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walter F, Schmid J, Düssmann H, Concannon CG, Prehn JH. Imaging of single cell responses to ER stress indicates that the relative dynamics of IRE1/XBP1 and PERK/ATF4 signalling rather than a switch between signalling branches determine cell survival. Cell Death Differ. 2015;22:1502–1516. doi: 10.1038/cdd.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo FJ, Liu Y, Zhou J, Luo S, Zhao W, Li X, Liu C. XBP1S protects cells from ER stress-induced apoptosis through Erk1/2 signaling pathway involving CHOP. Histochem Cell Biol. 2012;138:447–460. doi: 10.1007/s00418-012-0967-7. [DOI] [PubMed] [Google Scholar]
- 18.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CJ, Prazak L, Fajardo M, Yu S, Tyagi N, Di Cesare PE. Leukemia/lymphoma-related factor a POZ domain-containing transcriptional repressor, interacts with histone deacetylase-1 and inhibits cartilage oligomeric matrix protein gene expression and chondrogenesis. J Biol Chem. 2004;279:47081–47091. doi: 10.1074/jbc.M405288200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Kong L, Carlson CS, Liu CJ. Cbfa1-dependent expression of an interferon-inducible p204 protein is required for chondrocyte differentiation. Cell Death Differ. 2008;15:1760–1771. doi: 10.1038/cdd.2008.112. [DOI] [PubMed] [Google Scholar]
- 21.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: Cell survival and cell death. Int J Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo F, Lin EA, Liu P, Lin J, Liu C. XBP1U inhibits the XBP1S-mediated upregulation of the iNOS gene expression in mammalian ER stress response. Cell Signal. 2010;22:1818–1828. doi: 10.1016/j.cellsig.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 24.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI0216784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumley EC, Osborn AR, Scott JE, Scholl AG, Mercado V, McMahan YT, Coffman ZG, Brewster JL. Moderate endoplasmic reticulum stress activates a PERK and p38-dependent apoptosis. Cell Stress Chaperones. 2016 Oct 20; doi: 10.1007/s12192-016-0740-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah A, Kumar A. Methamphetamine-mediated endoplasmic reticulum (ER) stress induces type-1 programmed cell death in astrocytes via ATF6, IRE1α and PERK pathways. Oncotarget. 2016 Jun 14; doi: 10.18632/oncotarget.10025. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo FJ, Xiong Z, Han X, Liu C, Liu Y, Jiang R, Zhang P. XBP1S, a BMP2-inducible transcription factor, accelerates endochondral bone growth by activating GEP growth factor. J Cell Mol Med. 2014;18:1157–1171. doi: 10.1111/jcmm.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X, Zhou J, Zhang P, Song F, Jiang R, Li M, Xia F, Guo FJ. IRE1α dissociates with BiP and inhibits ER stress-mediated apoptosis in cartilage development. Cell Signal. 2013;25:2136–2146. doi: 10.1016/j.cellsig.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pincus D, Chevalier MW, Aragón T, van Anken E, Vidal SE, El-Samad H, Walter P. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 32.Samali A, Zhivotovsky B, Jones D, Nagata S, Orrenius S. Apoptosis: Cell death defined by caspase activation. Cell Death Differ. 1999;6:495–496. doi: 10.1038/sj.cdd.4400520. [DOI] [PubMed] [Google Scholar]
- 33.Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 37.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakanishi K, Sudo T, Morishima N. Endoplasmic reticulum stress signaling transmitted by ATF6 mediates apoptosis during muscle development. J Cell Biol. 2005;169:555–560. doi: 10.1083/jcb.200412024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hassler J, Cao SS, Kaufman RJ. IRE1, a double-edged sword in pre-miRNA slicing and cell death. Dev Cell. 2012;23:921–923. doi: 10.1016/j.devcel.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]