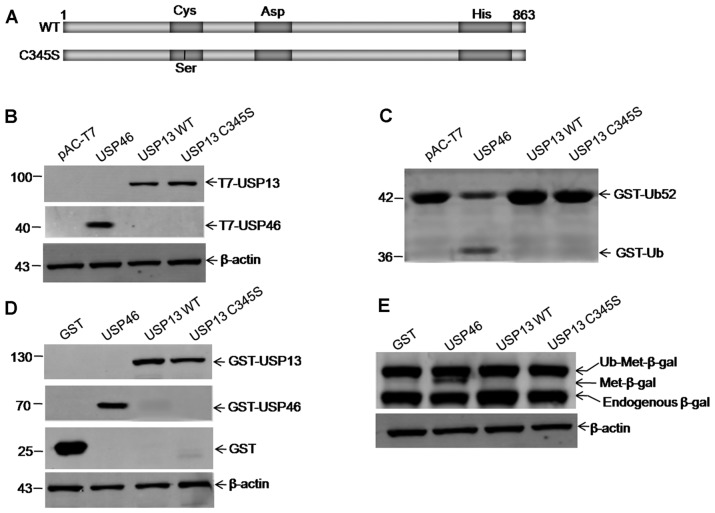
Figure 2.
Ubiquitin-specific protease 13 (USP13) exhibited no detectable activity for the hydrolysis of model substrates. (A) Constructs of wild-type (WT) and mutant USP13 C345S for the USP cleavage assay. (B) Expression of T7-USP13 and T7-USP46 can be detected by western blotting with anti-T7 antibody. (C) Cleavage of model substrate glutathione S-transferase (GST)-Ub52. The arrows indicate the GST-Ub52 (about 42 kDa) and its cleavage products GST-Ub (about 36 kDa), which were detected by 10% SDS-PAGE. USP46 and pAC-T7 (an empty plasmid vector) are used as positive and negative controls, respectively. (D) Total protein extracts were examined by western blot analysis with anti-GST to detect the expression of USPs. (E) Deubiquitination of Ub-Met-β-gal expressed in bacteria. Western blot analysis with anti-β-gal antiserum is shown. The arrows indicate the ubiquitin-β-galactosidase (Ub-Met-β-gal) fusion protein, the cleaved β-gal moiety (Met-β-gal), and endogenous β-gal fragments. Co-expressed plasmids were pGEX-USP13, the positive control pGEX–USP46, and the negative control pGEX-6P-1 (GST).