Figure 1.
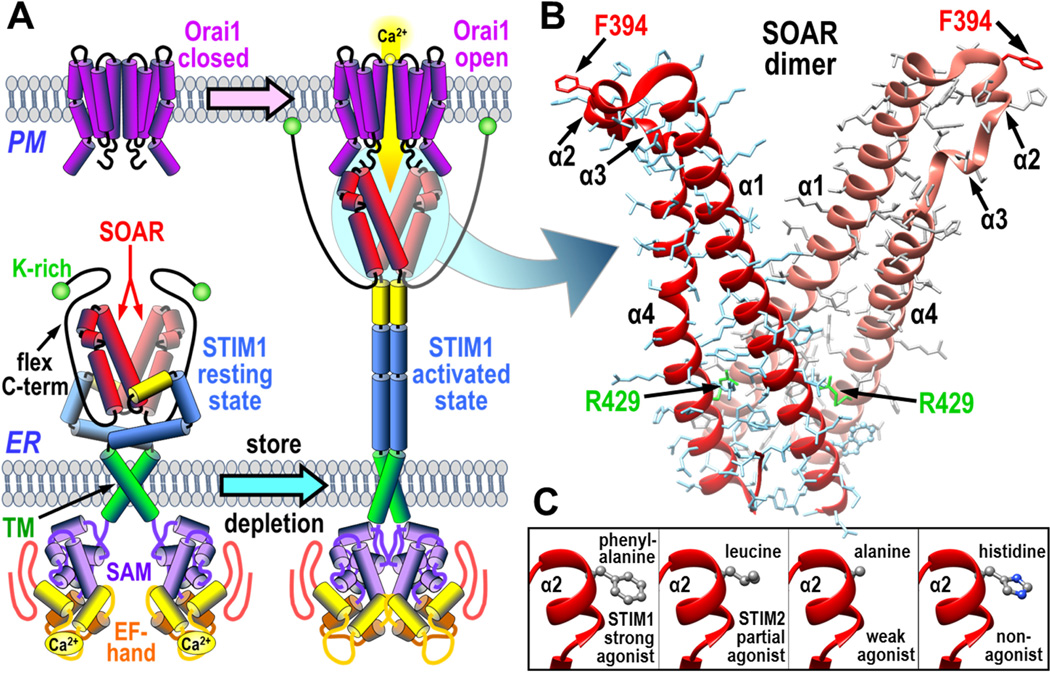
The coupling interface between STIM1 and Orai1. (A) Overall scheme for activation and coupling between the STIM1 dimer in the endoplasmic reticulum (ER) and Orai1 channel in the plasma membrane (PM). STIM1 is shown in its resting state (left) and activated state after store depletion and dissociation of luminal Ca2+ (right). The Orai1 channel is shown in its resting closed state (left) and activated open state coupled to the STIM1 protein (right). The STIM1 domains shown include the STIM-Orai activating region (SOAR), the lysine-rich C-terminus (K-rich), the flexible C-terminal domain (flex C-term), the transmembrane region (TM), the sterile-α motif (SAM), and the Ca2+-binding EF-hand segment. (B) Structure of dimeric SOAR domain from STIM1 revealing the four α-helical domains (α1, α2, α3, and α4), the active STIM1-binding site residue, Phe-394, and the structurally important Arg-429 residue. (C) Detail of the α2 domain of SOAR to show the wildtype Phe-394 residue, and substitutions of this residue with leucine (found in STIM2) or with alanine or histidine which block the interaction with Orai1.