Abstract
Ca2+ influx across the plasma membrane is a key component of the receptor-evoked Ca2+ signaling that mediate numerous cell functions and reload the ER after partial or full ER Ca2+ store depletion. Ca2+ influx is activated in response to Ca2+ release from the ER, a concept developed by Jim Putney, and the channels mediating the influx are thus called store-operated Ca2+ influx channels, or SOCs. The molecular identity of the SOCs has been determined with the identification of the TRPC channels, STIM1 and the Orai channels. These channels are targeted to, operate and are regulated when at the ER/PM junctions. ER/PM junctions are a form of membrane contact sites (MCSs) that are present in all parts of the cells, where the ER makes contacts with cellular membranes and organelles. MCSs have many cellular functions, and are the sites of lipid and Ca2+ transport and delivery between organelles. This short review discusses aspects of MCSs in the context of lipid and Ca2+ transport.
Graphical abstract
Introduction
Membrane contact sites (MCSs) are present in many cellular compartments. The endoplasmic reticulum (ER) forms most MCSs by tethering with the plasma membrane (PM), mitochondria, endosomes, lysosomes, peroxisomes, and in yeast with the vacuole [1–5]. Most information on the structure and function of MCSs came from studies of lipid synthesis and transfer in yeast, although similar information in mammalian cells is rapidly accumulating, as new tools for their studies become available (for recent reviews see [2, 4, 6]). MCSs have many functions in cell metabolism and signaling. MCSs is where lipid synthesis complexes and lipid transport proteins are located and where lipid synthesis and transport between organelles takes place [5, 6]. Many signaling proteins resides at MCSs or are recruited to MCSs during cell stimulation [2, 4, 6], as is the case with Orai1 and STIM1 [7–10]. Mitochondrial fission takes place at MCSs, as well as initiation of autophagy and mitophagy [2, 6]. In the case of Ca2+ signaling, the ER/PM junctions are the MCSs at which the STIM1-Orai1 and STIM1-TRPC channels complexes assemble to mediate the store-operated Ca2+ influx (SOCs) [10–12]. Ca2+ transfer between the ER and mitochondria takes place at the ER/mitochondria MCSs [13, 14].
The ER/PM MCSs
Examining lipid transfer between the ER and plasma membrane (PM) in yeasts identified several of the tether proteins at the ER/PM junctions. They include the three Tricalbins, the Increased sodium tolerance protein 2 (Ist2) and the ER resident Scs2/22 [1, 15]. Deletion of all six proteins was necessary to dissociate the ER/PM junctions in yeast [15]. The mammalian homologues of these proteins are known to a limited extent. The Tricalbins homologues are the three extended synaptotagmins (E-Syts) [16, 17], and the Scs2/22 homologues are VAP-A and VAP-B [4]. The yeast Tricalbins and the mammalian E-Syts have a synaptotagmin-like mitochondrial and lipid binding protein (SMP) domain [18, 19]. The SMP domain is present in many tether proteins, in various cellular compartments and was shown recently to mediate lipid transfer between bilayers [20, 21]. The homologue of Ist2 is not known yet, although Ist2 shows homology to the Anoctamins, a family of 10 proteins with diverse functions, including Ca2+-activated Cl− channels (ANO1 and ANO2) [22] and lipid scrambling (ANO6 and others) [23–25]. Whether Ist2 mediates lipid transfer, exchange or scrambling is not known.
Other proteins that may reside at the same or adjacent to the ER/PM junctions are the oxysterol-binding protein-related proteins (ORPs) ORP5 and ORP8 [26–28] and the lipid transfer proteins Nir2 and Nir3 [29, 30]. ORP5/8 mediate PS/PI4P exchange to deliver PS to the plasma membrane and PI4P to the ER [26–28]. Nir2 clusters at the ER/PM junction in response to cell stimulation and replenishes PM PI(4,5)P2 and PI(3,4,5)P3 [4, 29, 30]. An important function by Nir2 reported recently is transfers PI from the ER to the PM and at the same time transfer PA from the PM to the ER, suggesting that Nir2 functions as PI/PA exchanger [31]. The topics of lipid transfer and the structure and function of the ER/PM junctions are relatively new, at least in mammalian cells, and new components are continually being identified.
Ca2+ signals at MCSs
The best documented Ca2+ signaling at MCSs are Ca2+ signaling at the ER/mitochondria and Ca2+ influx by store operated Ca2+ channels (SOCs). Ca2+ signaling at the ER/mitochondria are discussed at length elsewhere [13, 32] and we have restricted our short comments to the function of the SOCs. The concept of SOCs was introduced to explain fluid and electrolyte secretion by secretory glands [33]. In the revised version of the hypothesis it was postulated that Ca2+ release from the ER in response to cell stimulation somehow results in activation of Ca2+ influx pathway at the plasma membrane [34]. At about the same time, we showed that Ca2+ influx across the plasma membrane in response to store depletion to reload the ER with Ca2+ takes place at free cytoplasmic Ca2+ concentration close to the resting levels [35, 36]. This is due to the high capacity of Ca2+ uptake by the sarco/endoplasmic reticulum (SR) calcium transport ATPase (SERCA) pump compared to the rate of Ca2+ influx by the SOCs [36]. The concept of SOCs then received decisive evidence with the demonstration that passive store depletion by inhibition of the SERCA pump with thapsigargin activated the same Ca2+ influx pathway as the receptor-stimulated depletion of ER Ca2+ [37]. The next step was recording the Ca2+-release activated current (CRAC) [38] and identification of the TRPC channels as receptor activated Ca2+ influx channels [39, 40], although the TRPCs did not mediate the CRAC current. The finding of STIM1 [9, 41] and Orai1 [42–44] and that STIM1-Orai1 complexes mediate the CRAC current [43, 45] established the molecular components of the SOCs. Clustering of STIM1, Orai1 and the TRPC channels at ER/PM junctions [11, 46] highlighted the importance of the ER/PM junction in SOCs channels function.
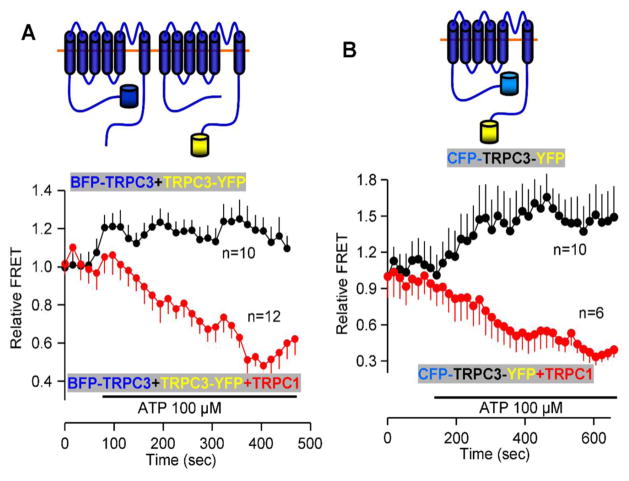
TRPC channels can function in both store-dependent and store-independent modes [47, 48]. When they function in a store-dependent mode, they are regulated by STIM1 [48–50], and are strictly dependent on the STIM1 polybasic, PI(4,5)P2 interacting domain and two conserved negative charges in the C terminus of the TRPC channels [49, 50]. Moreover, gating by STIM1 of several TRPC channels requires their interaction [47, 48]. An example is shown in Figure 1 in which TRPC1 interacts with TRPC3 to dissociate the interaction between the two TRPC3 coiled-coil domains [47], which is required for regulation of TRPC3 by STIM1 [49, 50]. For TRPC channels to be regulated by STIM1, they must be present at the ER/PM junctions where STIM1 clusters are found. Indeed, extensive studies showed the recruitment of TRPC1 to the STIM1 puncta [11] a process facilitated by STIM2 [51]. In native cells STIM1, Orai1 TRPC1 [52] and TRPC3 [53] are found at the sites at which STIM1 is recruited by store depletion to form complexes that can be immunoprecipitated after ER Ca2+ store depletion [52].
Figure 1. TRPC1 dissociates TRPC3 N and C terminal coiled-coil domains.
(A) BFP-TRPC3 and TRPC3-YFP were co-expressed alone of together with TRPC1 in HEK cells. Cell stimulation slightly increased TRPC3-TRPC3 interaction in the absence of TRPC1 (black symbols and trace), while TRPC1 caused dissociation of interacting TRPC3 monomers (red symbols and trace). (B) similar experiment as in (A) except that TRPC3 was double tagged with BFP at the N terminus and YFP at the C terminus. In this case, TRPC1 dissociates the interaction between the same molecule TRPC3 N and C termini coiled-coil domains.
The properties and mechanism of activation of Orai1 by STIM1 is discussed elsewhere in this special issue. For the current discussion, it should be noted that STIM1 fulfills all the properties of a tether protein. STIM1 has N-terminal transmembrane domain that spans the ER membrane, a long cytoplasmic domain that bridges the distance between the ER and PM and a C terminal polybasic domain that binds PI(4,5)P2 at the PM. At the junctions, STIM1 clusters both Orai1 and the TRPC channels. The ER/PM junctions at which STIM1 clusters are formed by tether proteins. Establish tethered are E-Syt1 [10, 17] and Nir2/3 [4, 30, 31]. Another likely tether is a mammalian homologue of Ist2 that can be one of the ANO proteins.
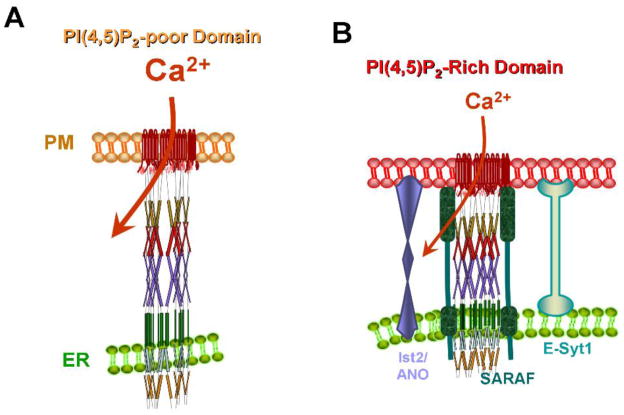
Localization of the STIM1-Orai1 complex at the ER/PM junctions defines the Ca2+ entry sites at the plasma membrane. Moreover, the STIM1-Orai1 complex activity is specifically regulated by Ca2+ only when it is at the ER/PM junctions and is experiencing high levels of PI(4,5)P2 [10]. This is illustrated in Figure 2. Ca2+ influx by Orai1 is inhibited by high Ca2+ in two modes, fast Ca2+-dependent inhibition that is completed in 100 msec and slow Ca2+-dependent inhibition (SCDI) that is completed in 1–2 min [54]. Both forms of inhibition are mediated by the ER resident protein SARAF [10, 55]. Ca2+-dependent inhibition (CDI) requires stabilization of the ER/PM junctions by E-Syt1, binding of the STIM1 polybasic domain to PI(4,5)P2 and presence of the STIM1-Orai1 complex in a PI(4,5)P2-rich plasma membrane domain [10] (Figure 2). Whether other lipids are also enriched at the ER/PM junctions and are required for optimal functioning of the STIM1-Orai1 complex remains to be determined.
Figure 2. STIM1-Orai1 complex at PI(4,5)P2 poor and rich ER/PM junctions.
(A) Upon store depletion STIM1 and Orai1 cluster at PI(4,5)P2-poor domain where Ca2+ influx is maximally active. (B) translocation of STIM1-Orai1 to a PI(4,5)P2-rich domain results in recruitment of SARAF that interacts with STIM1 to mediate inhibition of Ca2+ influx by Orai1.
The identity, properties and function of all tethers at mammalian cell ER/PM junctions are not known and are likely to be further defined in the near future. It is also likely that diverse ER/PM junctions with specific properties are dedicated to specific cellular function. A clue may be provided in the finding that E-Syt1, but not E-Syt2 and E-Syt3, are required for CDI by SARAF [10], while E-Syt2, but not E-Syt1 and E-Syt3 are required for synthesis of PI4P [56]. The specificity and relationship between the various ER/PM junctions need to be explored.
Highlights.
Tethers at the ER/PM junctions
STIM1-TRPC complexes at the ER/PM junctions
STIM1-Orai1 complexes at the ER/PM junctions
Footnotes
All authors declare no conflict of interests and this manuscript was not submitted elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lahiri S, Toulmay A, Prinz WA. Membrane contact sites, gateways for lipid homeostasis. Current opinion in cell biology. 2015;33:82–87. doi: 10.1016/j.ceb.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips MJ, Voeltz GK. Structure and function of ER membrane contact sites with other organelles. Nature reviews Molecular cell biology. 2016;17:69–82. doi: 10.1038/nrm.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong LH, Levine TP. Lipid transfer proteins do their thing anchored at membrane contact sites... but what is their thing? Biochemical Society transactions. 2016;44:517–527. doi: 10.1042/BST20150275. [DOI] [PubMed] [Google Scholar]
- 4.Selitrennik M, Lev S. The role of phosphatidylinositol-transfer proteins at membrane contact sites. Biochemical Society transactions. 2016;44:419–424. doi: 10.1042/BST20150182. [DOI] [PubMed] [Google Scholar]
- 5.Kentala H, Weber-Boyvat M, Olkkonen VM. OSBP-Related Protein Family: Mediators of Lipid Transport and Signaling at Membrane Contact Sites. International review of cell and molecular biology. 2016;321:299–340. doi: 10.1016/bs.ircmb.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Murray JP, McMaster CR. Lipid synthesis and membrane contact sites: a crossroads for cellular physiology. Journal of lipid research. 2016;57:1789–1805. doi: 10.1194/jlr.R070920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. The Journal of cell biology. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perni S, Dynes JL, Yeromin AV, Cahalan MD, Franzini-Armstrong C. Nanoscale patterning of STIM1 and Orai1 during store-operated Ca2+ entry. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E5533–5542. doi: 10.1073/pnas.1515606112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maleth J, Choi S, Muallem S, Ahuja M. Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nature communications. 2014;5:5843. doi: 10.1038/ncomms6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong HL, Ambudkar IS. Molecular determinants of TRPC1 regulation within ER-PM junctions. Cell calcium. 2015 doi: 10.1016/j.ceca.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Cao X, Choi S, Maleth JJ, Park S, Ahuja M, Muallem S. The ER/PM microdomain, PI(4,5)P and the regulation of STIM1-Orai1 channel function. Cell calcium. 2015 doi: 10.1016/j.ceca.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filadi R, Pozzan T. Generation and functions of second messengers microdomains. Cell calcium. 2015;58:405–414. doi: 10.1016/j.ceca.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Booth DM, Enyedi B, Geiszt M, Varnai P, Hajnoczky G. Redox Nanodomains Are Induced by and Control Calcium Signaling at the ER-Mitochondrial Interface. Molecular cell. 2016;63:240–248. doi: 10.1016/j.molcel.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Developmental cell. 2012;23:1129–1140. doi: 10.1016/j.devcel.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Min SW, Chang WP, Sudhof TC. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3823–3828. doi: 10.1073/pnas.0611725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopec KO, Alva V, Lupas AN. Homology of SMP domains to the TULIP superfamily of lipid-binding proteins provides a structural basis for lipid exchange between ER and mitochondria. Bioinformatics. 2010;26:1927–1931. doi: 10.1093/bioinformatics/btq326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopec KO, Alva V, Lupas AN. Bioinformatics of the TULIP domain superfamily. Biochemical Society transactions. 2011;39:1033–1038. doi: 10.1042/BST0391033. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Liu Y, Gulbranson DR, Paine A, Rathore SS, Shen J. Extended synaptotagmins are Ca2+-dependent lipid transfer proteins at membrane contact sites. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:4362–4367. doi: 10.1073/pnas.1517259113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saheki Y, Bian X, Schauder CM, Sawaki Y, Surma MA, Klose C, Pincet F, Reinisch KM, De Camilli P. Control of plasma membrane lipid homeostasis by the extended synaptotagmins. Nature cell biology. 2016;18:504–515. doi: 10.1038/ncb3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh U, Jung J. Cellular functions of TMEM16/anoctamin. Pflugers Archiv : European journal of physiology. 2016;468:443–453. doi: 10.1007/s00424-016-1790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki J, Fujii T, Imao T, Ishihara K, Kuba H, Nagata S. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. The Journal of biological chemistry. 2013;288:13305–13316. doi: 10.1074/jbc.M113.457937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 25.Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature. 2014;516:207–212. doi: 10.1038/nature13984. [DOI] [PubMed] [Google Scholar]
- 26.Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P. INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 2015;349:428–432. doi: 10.1126/science.aab1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moser von Filseck J, Copic A, Delfosse V, Vanni S, Jackson CL, Bourguet W, Drin G. INTRACELLULAR TRANSPORT. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science. 2015;349:432–436. doi: 10.1126/science.aab1346. [DOI] [PubMed] [Google Scholar]
- 28.Sohn M, Ivanova P, Brown HA, Toth DJ, Varnai P, Kim YJ, Balla T. Lenz-Majewski mutations in PTDSS1 affect phosphatidylinositol 4-phosphate metabolism at ER-PM and ER-Golgi junctions. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:4314–4319. doi: 10.1073/pnas.1525719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang CL, Liou J. Phosphatidylinositol 4,5-Bisphosphate Homeostasis Regulated by Nir2 and Nir3 Proteins at Endoplasmic Reticulum-Plasma Membrane Junctions. The Journal of biological chemistry. 2015;290:14289–14301. doi: 10.1074/jbc.M114.621375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang CL, Hsieh TS, Yang TT, Rothberg KG, Azizoglu DB, Volk E, Liao JC, Liou J. Feedback regulation of receptor-induced Ca2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell reports. 2013;5:813–825. doi: 10.1016/j.celrep.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 31.Kim YJ, Guzman-Hernandez ML, Wisniewski E, Balla T. Phosphatidylinositol-Phosphatidic Acid Exchange by Nir2 at ER-PM Contact Sites Maintains Phosphoinositide Signaling Competence. Developmental cell. 2015;33:549–561. doi: 10.1016/j.devcel.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnoczky G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Molecular cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putney JW., Jr A model for receptor-regulated calcium entry. Cell calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Putney JW., Jr Capacitative calcium entry revisited. Cell calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 35.Pandol SJ, Schoeffield MS, Sachs G, Muallem S. Role of free cytosolic calcium in secretagogue-stimulated amylase release from dispersed acini from guinea pig pancreas. The Journal of biological chemistry. 1985;260:10081–10086. [PubMed] [Google Scholar]
- 36.Muallem S, Fimmel CJ, Pandol SJ, Sachs G. Regulation of free cytosolic Ca2+ in the peptic and parietal cells of the rabbit gastric gland. The Journal of biological chemistry. 1986;261:2660–2667. [PubMed] [Google Scholar]
- 37.Takemura H, Hughes AR, Thastrup O, Putney JW., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. The Journal of biological chemistry. 1989;264:12266–12271. [PubMed] [Google Scholar]
- 38.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 39.Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS letters. 1995;373:193–198. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- 41.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. The Journal of cell biology. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 43.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nature cell biology. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin DM, Son A, Park S, Kim MS, Ahuja M, Muallem S. The TRPCs, Orais and STIMs in ER/PM Junctions. Advances in experimental medicine and biology. 2016;898:47–66. doi: 10.1007/978-3-319-26974-0_3. [DOI] [PubMed] [Google Scholar]
- 47.Lee KP, Choi S, Hong JH, Ahuja M, Graham S, Ma R, So I, Shin DM, Muallem S, Yuan JP. Molecular determinants mediating gating of Transient Receptor Potential Canonical (TRPC) channels by stromal interaction molecule 1 (STIM1) The Journal of biological chemistry. 2014;289:6372–6382. doi: 10.1074/jbc.M113.546556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nature cell biology. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Molecular cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee KP, Yuan JP, So I, Worley PF, Muallem S. STIM1-dependent and STIM1-independent Function of Transient Receptor Potential Canonical (TRPC) Channels Tunes Their Store-operated Mode. The Journal of biological chemistry. 2010;285:38666–38673. doi: 10.1074/jbc.M110.155036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ong HL, de Souza LB, Zheng C, Cheng KT, Liu X, Goldsmith CM, Feske S, Ambudkar IS. STIM2 enhances receptor-stimulated Ca(2)(+) signaling by promoting recruitment of STIM1 to the endoplasmic reticulum-plasma membrane junctions. Sci Signal. 2015;8:ra3. doi: 10.1126/scisignal.2005748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, Cheng KT, Ambudkar IS, Muallem S. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic. 2011;12:232–245. doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. The Journal of biological chemistry. 2006;281:32540–32549. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- 54.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 55.Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E. SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell. 2012;149:425–438. doi: 10.1016/j.cell.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 56.Dickson EJ, Jensen JB, Vivas O, Kruse M, Traynor-Kaplan AE, Hille B. Dynamic formation of ER-PM junctions presents a lipid phosphatase to regulate phosphoinositides. J Cell Biol. 2016;213:33–48. doi: 10.1083/jcb.201508106. [DOI] [PMC free article] [PubMed] [Google Scholar]