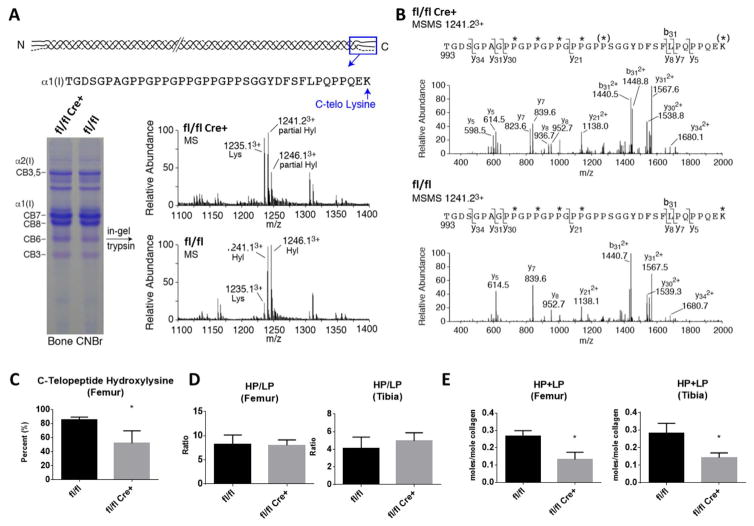
Figure 4. Post-translational Collagen Modification and Cross-linking.
(A) SDS-PAGE of CNBr digested bone and mass spectral analysis of in-gel trypsin digested α1(I) CB6. The C-terminal tryptic peptide shown reveals the α1(I) C-telopeptide lysine hydroxylation state. (B) MS/MS fragmentation spectra of parent ion (1241.23+) from fl/fl and fl/fl Cre+ bone α1(I). The fl/fl Cre+ spectrum shows both lysine and hydroxylysine fragment ions, whereas only hydroxylysine fragment ions are present in the control. (C) Quantitation of bone collagen C-telopeptide lysine hydroxylation by mass spectrometry. (D) The hydroxylysylpyridinoline/lysylpyridinoline (HP/LP) ratio was not altered in mutant compared to control littermate bones, but the total crosslinks (HP+LP) were significantly reduced in both the femur and tibia (E). Statistical analyses were performed by Student’s t-test for each parameter. N=3–4 per group, values in mean ± SD, *p<0.05.