Figure 2.
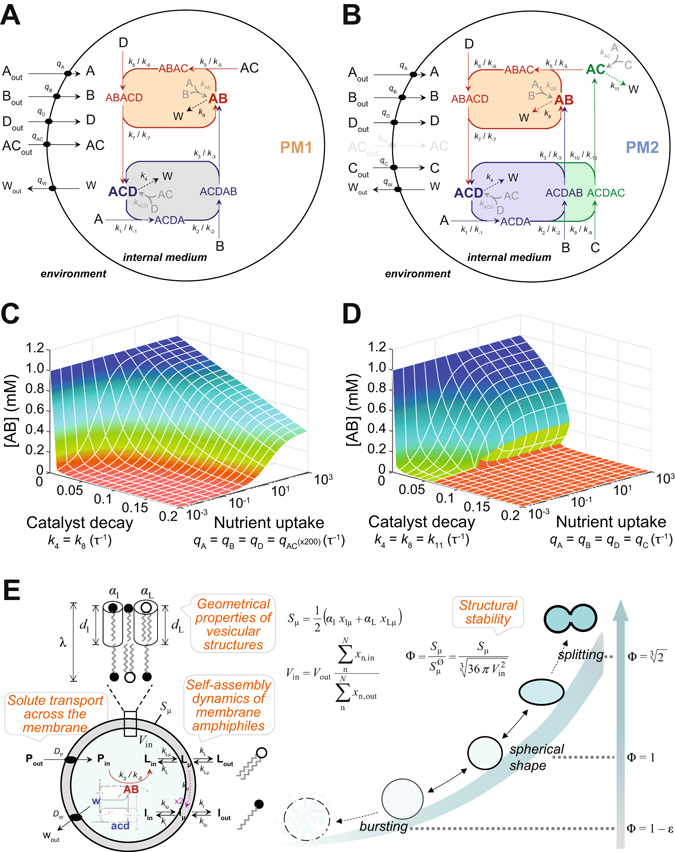
Coupling between primitive compartments and metabolisms: protocell modeling assumptions and physical-chemical constraints. (A,B) Two minimal models of protometabolism are considered: PM1 (A), which is formed by two mutually-promoting catalytic cycles driven by the AB and ACD species; and PM2 (B), containing one extra reinforcing cycle leading to the AC species. Both of them rely on the uptake of the corresponding energy-rich precursors from the environment and on rudimentary catalysts of limited lifespan (decay reactions shown as dashed arrows). (C,D) The build-up of metabolites within a membrane compartment, represented by the steady-state concentration of the species AB, is sensitive to both the decay rates of catalysts and the accessibility to precursors (i.e. nutrient permeability through the membrane). These factors influenced PM1 (C) and PM2 (D) differently, depending on the needs and costs of each metabolic network architecture. (E) The L lipid species was assumed to be synthesized internally from a given diffusible precursor P, in a reaction catalyzed by AB. L contributed to increase the overall protocell surface area Sμ and displaced (more or less efficiently, depending on k d) the naturally-occurring l lipid molecules within the membrane bilayer. In addition, some end-products of the protometabolic activity (the permeable w species and the totally impermeable acd species) tend to accumulate within the protocell, inducing volume growth (through a simulated water inflow–to keep the isotonic condition). The actual balance between vesicular surface area and internal volume V in determined the protocell stability and, ultimately, its propensity to undergo splitting or bursting (see main Text and SI).
