Abstract
Nicotinic acetylcholine receptors (nAChRs) in the mesolimbic dopamine system have been implicated in ethanol behaviors. In particular, work in genetically engineered mice has demonstrated that α6-containing nAChRs are involved in ethanol consumption and sedation. A limitation of these studies is that the alteration in the receptor was present throughout development. The recently described α6β2 antagonist, N,N-decane-1,10-diyl-bis-3-picolinium diiodide (bPiDI), now makes it possible to test for the involvement of these receptors using a pharmacological approach. The aim of this study was to examine the role of α6β2 nAChRs in ethanol behaviors using a pharmacological approach. Adolescent C57BL/6J mice were treated with bPiDI 30 minutes prior to testing the mice for binge-like ethanol consumption in the drinking-in-the-dark (DID) test, ethanol-induced motor incoordination using the balance beam, and ethanol-induced sedation using the Loss of Righting Reflex (LORR) paradigm. Adolescent animals were chosen because they express a high amount of α6 mRNA relative to adult animals. Control studies were also performed to determine the effect of bPiDI on locomotor activity and ethanol metabolism. Female mice treated with 20 mg/kg bPiDI had reduced locomotor activity compared to saline-treated animals during the first 30 minutes following an acute injection. Pretreatment with the α6β2 antagonist reduced adolescent ethanol consumption but also reduced saccharin consumption. No significant effects were observed on ethanol-induced ataxia, sedation, or metabolism. This study provides evidence that α6β2 nAChRs are involved in locomotor activity as well as ethanol and saccharin consumption in adolescent animals.
Keywords: Ethanol, Nicotinic Acetylcholine Receptors, Ataxia, Sedation, Consumption, Chrna6
Introduction
Alcohol use disorders are common in the United States with a total lifetime prevalence of 30% (Hasin, Stinson, Ogburn, & Grant, 2007). Several medications are currently used to treat alcohol use disorders, but further research is needed to identify additional drugs that can alleviate this disease. Nicotinic acetylcholine receptors (nAChRs) have been implicated in many of ethanol’s behavioral responses including: consumption (Feduccia, Simms, Mill, Yi, & Bartlett, 2014; Hendrickson, Zhao-Shea, Pang, Gardner, & Tapper, 2010; Kamens, Andersen, & Picciotto, 2010a; Steensland, Simms, Holgate, Richards, & Bartlett, 2007), reward (Liu, Zhao-Shea, McIntosh, & Tapper, 2013; Powers, Broderick, Drenan, & Chester, 2013), ataxia (Kamens, Andersen, & Picciotto, 2010b; Taslim, Al-Rejaie, & Saeed Dar, 2008), sedation (Bowers et al., 2005; Kamens et al., 2010b; Kamens, Hoft, Cox, Miyamoto, & Ehringer, 2012), and locomotor activation (Kamens et al., 2009; Kamens & Phillips, 2008; Larsson, Svensson, Söderpalm, & Engel, 2002). These data provide a compelling argument that this receptor system should be further examined in terms of treatment potential.
Data from both human genetic studies and animal models has provided evidence that the α6 nAChR subunit is involved in ethanol responses. Studies examining common genetic variation in CHRNA6 (the gene that codes for the α6 subunit) have shown that variation in this gene is associated with alcohol consumption (Hoft et al., 2009; Landgren et al., 2009). Moreover, recent work suggests that rare genetic variation in this region may be associated with risk of alcohol dependence (Haller et al., 2014).
Animal models have also shown a role for the α6 subunit in ethanol behaviors, but the results of these studies are mixed. Mice that lack the α6 subunit do not differ in ethanol consumption compared to wildtype animals (Guildford, Sacino, & Tapper, 2016; Kamens et al., 2012), but do differ in sensitivity to the sedative effects of ethanol (Kamens et al., 2012) and sensitivity to ethanol reward at high doses (Guildford et al., 2016). In contrast, transgenic mice that exhibit a hypersensitive α6 subunit consumed significantly more ethanol than wildtype animals and displayed ethanol reward at low doses (Powers et al., 2013). These mice were created using a bacterial artificial chromosome to express an α6 subunit that has a point mutation at the 9′ position that results in α6-containing nicotinic receptors that are hypersensitive to acetylcholine compared to the wildtype receptor (Drenan et al., 2008). Research with genetically engineered mice is not without important limitations. The primary limitation of both models is that the α6 subunit was either removed or altered during development, and compensatory effects of other nAChRs may have occurred. These data suggest that α6-containing nicotinic receptors may be involved in the behavioral effects of ethanol, but further research is needed.
The α6 subunit requires a β subunit (β2 or β3) to form functional receptors. In almost all known nAChRs α6 combines with a β2 subunit to form a functional receptor, although other subunits also co-assemble (Quik, Perez, & Grady, 2011). Until recently, no drugs that cross the blood brain barrier and specifically target α6-containing nAChRs have been available. Recently, a novel α6β2 nAChR antagonist, N,N-decane-1,10-diyl-bis-3-picolinium diiodide (bPiDI), has been described (Wooters et al., 2011). These receptors are the major subtype of nAChRs found in the nucleus accumbens which is a key brain region in modulating drug use (Quik, Perez, & Grady, 2011). Recent data in adult rats suggests that bPiDI can selectively decrease ethanol consumption (Srisontiyakul, Kastman, Krstew, Govitrapong, & Lawrence, 2016). To extend this line of research, we examined the role of α6β2 nAChRs in multiple ethanol behaviors in C57BL/6J mice. We examined the effect of bPiDI on: ethanol consumption, ethanol-induced ataxia, and ethanol-induced sedation. Based on the recent work of Srisontiyakul et al (2016), we hypothesized that a blockade of α6β2 nAChRs with bPiDI would decrease ethanol consumption. Two other hypotheses were developed based on our earlier work with genetically engineered mice (Kamens et al., 2012). We hypothesized that bPiDI would increase the sedative effects of ethanol, but have no effect on ethanol-induced motor coordination. We focused specifically on adolescent animals because of the high expression of the α6 subunit during this time (Azam, Chen, & Leslie, 2007).
Materials and Methods
Animals
Adolescent C57BL/6J mice purchased from The Jackson Laboratory (Bar Harbor, Maine) were used in all experiments. Mice arrived at 25 days of age, were housed in standard shoebox cages and had ad libitum access to food and water. Upon arrival, mice designated for drinking studies were singly housed, but animals for all other studies were housed in same sex groups of 2–4 per cage. Mice acclimated to either a standard 12 hour light/dark cycle (lights on at 0700) or a modified 12 hour light/dark cycle (lights on at 2200) for one week prior to testing. All procedures complied with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
Drugs
Ethyl alcohol (200 proof; Koptec) was used for all experiments. The ethanol was diluted in physiological saline (0.9% NaCl; Baxter) for injections (20% v/v; i.p.) or tap water for drinking solutions. N,N-decane-1,10-diyl-bis-3-picolinium diiodide (bPiDI) was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in saline for injections (i.p.). Saccharin sodium salt was obtained from Sigma-Aldrich (St. Louis, MO) and diluted in tap water for drinking studies.
Locomotor Activity
To investigate the effect of bPiDI on locomotor activity, 29 (15 female, 14 male) animals were tested at 32–33 days of age in a two day protocol similar to those published previously (Kamens et al., 2010b). Briefly, mice were tested in ten plastic arenas (19″ X 10.5″ X 8″, L X W X H, respectively). On the first day, animals were brought into the behavior room and allowed to acclimate for 45 minutes before testing. Animals were then injected with saline and placed into a test arena for 60 minutes. On the second day, mice were allowed to acclimate to the behavioral room for 45 minutes before they were injected with either saline or (15 or 20 mg/kg) bPiDI and immediately placed into a locomotor chamber for one hour. Locomotor activity was assessed by line crosses scored by two raters blind to treatment conditions.
Drinking in the Dark (DID)
To examine the effect of bPiDI on ethanol consumption, we utilized a 2 day drinking-in-the-dark (DID) procedure (Gupta et al., 2008; Kamdar et al., 2007). Singly housed mice (N = 24; 12 female and 12 male) went through four 2-day DID session separated by 1–2 days. Briefly, animals began testing at 32 days of age. The DID sessions occurred when mice were 32–33, 36–37, 39–40, and 43–44 days old. On day 1, water bottles were removed and a single 20% ethanol tube was provided at 3 hours into the dark cycle (Rhodes, Best, Belknap, Finn, & Crabbe, 2005). The volume of fluid in the tube (read to the closest 0.1 ml) was recorded immediately upon placement and at 30 min, 1 hour, and 2 hours. After the 2 hour exposure period, the ethanol tube was removed and water was returned. On Day 2, this was repeated with the exception that the animals were treated with an acute i.p. injection of saline or bPiDI (10, 15, or 20 mg/kg), 30 minutes prior to the 2 hour ethanol access period. All mice received each dose of bPiDI in a Latin square design. To determine if the effects on ethanol consumption were specific, a separate group of animals (N = 24) went through the same procedure, but had access to 0.033% saccharin instead of ethanol (Kamens et al., 2010a). For both ethanol and saccharin consumption, two control cages with no animal were handled with the same procedure as the experimental cages. The average leakage from these control cages was subtracted from drinking values obtained for experimental animals.
Balance Beam
To examine the effect of bPiDI on the ataxic effects of ethanol, naïve male and female mice were tested at 32–33 days of age using a balance beam (Crabbe et al., 2003; Kamens et al., 2010b; Linsenbardt et al., 2009). On day 1 all mice were given two training sessions, each of which consisted of a single crossing on an acrylic balance beam (¾ inch wide) suspended at a height of 54.6 cm off of the ground. On the second day of testing, mice were treated with an i.p. injection of saline or bPiDI (10, 15, or 20 mg/kg) and placed in a holding cage for 30 minutes. The mice were then challenged with saline or 1.5 g/kg ethanol before being placed back into the holding cage. Ten minutes after the challenge injection, the mice were placed on the balance beam and the number of hindpaw slips were counted as the mouse crossed the beam by an experimenter blind to the animal’s injection.
LORR
To examine the effect of bPiDI on the sedative-hypnotic effects of ethanol the Loss of Righting Reflex (LORR) was measured (Crabbe, Metten, Ponomarev, Prescott, & Wahlsten, 2006; Kamens et al., 2010b, 2012). Male and female mice were tested at 39 days old, 7 days after being tested on the balance beam. Mice received a pre-injection of saline, 10, 15, or 20 mg/kg bPiDI and were placed in a holding cage and left undisturbed for 30 minutes before being challenged with a 4.0 g/kg ethanol injection. Mice were then placed into a cage until they appeared intoxicated (approximately 1 minute) and were placed on their back in a V-shaped acrylic trough. Mice were determined to have lost their righting reflex when they were unable to right themselves from a supine position for at least 30 seconds. Mice were removed if they did not lose their righting reflex within 3 minutes of injection as evidence of a misplaced injection (Ponomarev & Crabbe, 2002). Time to LORR was recorded as time from injection until the animal lost its righting reflex. Mice were then observed until they succeeded in righting themselves twice in one minute, and this was recorded as duration of LORR.
Ethanol Metabolism
To determine the impact of bPiDI on ethanol metabolism, an established procedure was used (Kamens et al., 2006, 2010a). Mice that had previously undergone locomotor testing were allowed one week to rest and were tested at 43 or 44 days of age for ethanol metabolism. Briefly, mice were moved to the test room, weighed, and left undisturbed to acclimate to the room for at least 1 hour. Mice were treated with an acute injection of saline or bPiDI (15 or 20 mg/kg) before being placed into a holding cage. After 30 minutes all mice were given an i.p. injection of 4 g/kg ethanol. A 10 μL blood sample was taken from the tail vein 30, 60, 120, and 180 minutes following the ethanol injection. Blood ethanol concentrations were measured as previously described (Ehringer, Hoft, & Zunhammer, 2009; Kamens et al., 2012).
Statistical Analysis
Line crosses, ethanol consumption, saccharin consumption, footslips, time to LORR, duration of LORR, and BEC were used as primary dependent variables. Independent factors included sex and time. A repeated measures analysis of variance (ANOVA) was used to analyze data from the DID and metabolism studies, while all other studies were analyzed with a factorial ANOVA. Significant effects were followed with Tukey’s HSD for post hoc comparisons. α < 0.05 was considered significant.
Results
Locomotor activity
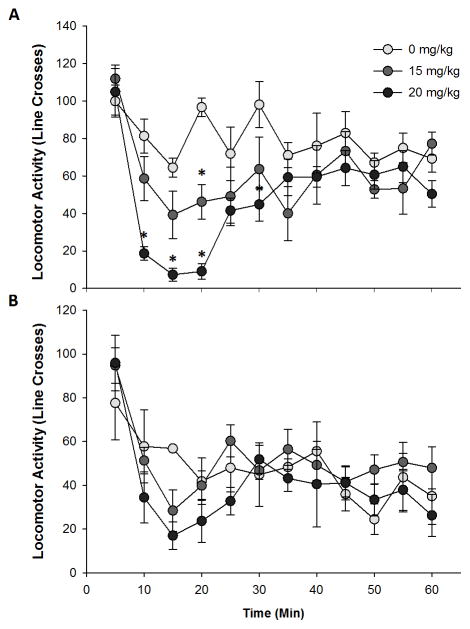
bPiDI transiently reduced locomotor activity in female C57BL/6J mice (Fig. 1). When locomotor activity across time was examined with a 2-way ANOVA, there was a significant main effect of sex (F1, 23=10.6, p<0.01) and sex X time interaction (F11, 253=2.1, p<0.05), so males and females were analyzed independently. In female mice, there was a significant main effect of time (F11, 132=12.2, p<0.001), dose (F2, 12=6.0, p<0.05), and time X dose interaction (F22, 132=3.6, p<0.001). There was a significant difference in locomotor activity at 10 (F2, 12=12.8, p<0.01), 15 (F2, 12=12.4, p<0.01), 20 (F2, 12=45.9, p<0.001), 30 (F2, 12=4.2, p<0.05), and 60 minutes (F2, 12=4.0, p<0.05). Specifically, the 20 mg/kg bPiDI dose significantly decreased locomotor activity between 10 – 30 minutes after the injection compared to saline treatment (p’s<0.05; Fig. 1A). The 15 mg/kg dose also reduced locomotor activity, but was only significantly less than saline at the 20 minute time point (p<0.01). At 60 minutes, the 20 mg/kg dose group was significantly different compared to the 15 mg/kg group, but not the saline group (p<0.05). In male mice, there was a significant main effect of time (F11, 121=9.6, p<0.001), such that activity was greatest during the first 5 minutes then decreased for the remainder of the test session (p<0.05; Fig. 1B). In male mice, there were no significant effects or interactions with dose observed.
Fig 1. bPiDI decreases locomotor activity.
Data (mean ± SEM) represent line crosses in female (A) and male (B) mice. N = 4 – 5 animals per group. Asterisks, significantly different from the control group.
DID
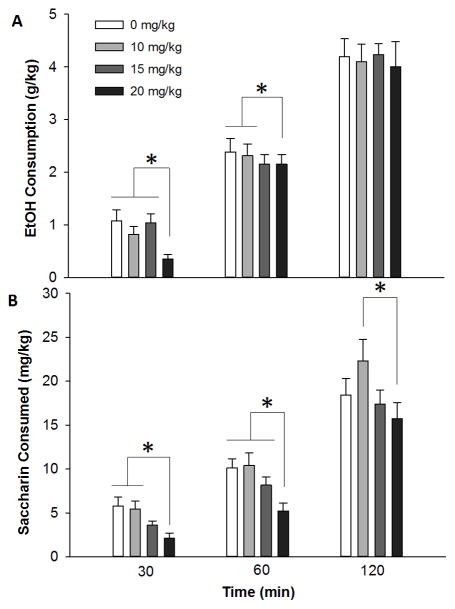
bPiDI decreased binge-like ethanol consumption in adolescent C57BL/6J mice (Fig 2A). Ethanol consumption (g/kg) was examined after 30, 60 and 120 minutes. Separate 1-way repeated measures AVNOVAs were performed for each DID time point, because of known time-dependent effects of nicotinic acetylcholine receptors on ethanol consumption (Kamens et al., 2010a; Steensland et al., 2007). At 30 minutes a significant main effect of dose (F3, 66=4.7, p<0.01) was detected. The high dose of bPiDI (20 mg/kg) significantly reduced ethanol consumption compared to saline, 10, or 15 mg/kg bPiDI (p’s<0.05). At 60 minutes the 20 mg/kg of bPiDI continued to reduce ethanol consumption compared to the saline or 10 mg/kg bPiDI dose as indicated by a significant main effect of dose (F3, 66=4.6, p<0.01). At 120 minutes, there were no significant effects of bPiDI on ethanol consumption.
Fig 2. bPiDI decreased binge-like ethanol consumption and saccharin consumption.
Data (mean ± SEM) represent ethanol consumption (A) and saccharin consumption (B). No significant main effects or interactions with sex were observed, so male and female data are combined. N = 24 animals per dose. Asterisks, p < 0.05.
Saccharin Consumption
To determine if the reduction in ethanol consumption was specific, we examined the effect of bPiDI on saccharin consumption using the DID paradigm. bPiDI reduced saccharin consumption in adolescent C57BL/6J mice (Fig 2B), indicating the that effect of this drug on ethanol consumption was not specific. At 30 minutes there was a significant main effect of dose (F3, 66=5.7, p<0.01) on saccharin consumption, such that the highest dose of bPiDI (20 mg/kg) reduced saccharin consumption compared to both saline and the 10 mg/kg bPiDI dose (p’s<0.05). At 60 minutes the high dose of bPiDI (20 mg/kg) significantly (F3, 66=7.4, p<0.01) reduced saccharin consumption compared to saline, 10 or 15 mg/kg bPiDI (p’s<0.05). At 120 minutes there was a significant main effect of dose (F3, 66=3.2, p<0.05), but at this time point the 20 mg/kg dose only reduced saccharin consumption compared to mice that received 10 mg/kg bPiDI (p<0.05).
Balance Beam
bPiDI did not influence ethanol-induced ataxia (Fig. 3). Treatment with bPiDI had no significant effect on footslips following a saline injection (p = 0.11; saline 0.33 ± 0.19; 10 mg/kg 0.33 ± 0.14; 15 mg/kg 0.92 ± 0.26; 20 mg/kg 1.0 ± 0.35 hindpaw slips). Thus, for further analyses, ethanol footslips were corrected by the average number of footslips made in the corresponding saline group that received the same dose of bPiDI (Crabbe et al., 2003; Kamens et al., 2010b). This corrected score shows the change in ethanol-induced ataxia while controlling for effects of saline and was used as the primary dependent variable. No significant main effects or interactions were observed on ethanol-induced ataxia.
Fig 3. bPiDI does not modulate ethanol-induced ataxia.
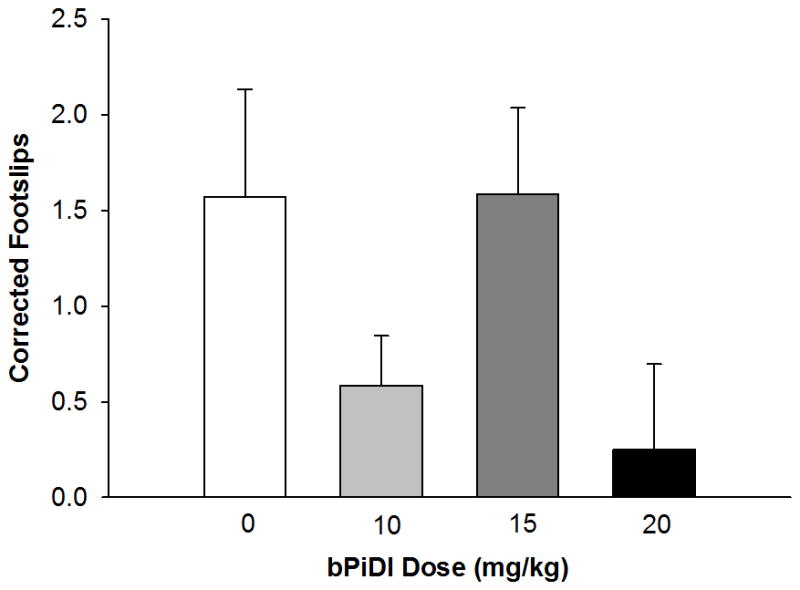
Data (mean ± SEM) represent corrected footslips (ethanol slips – baseline slips). No significant main effects or interactions with sex were observed, so male and female data are combined. N = 12 – 13 animals per dose.
LORR
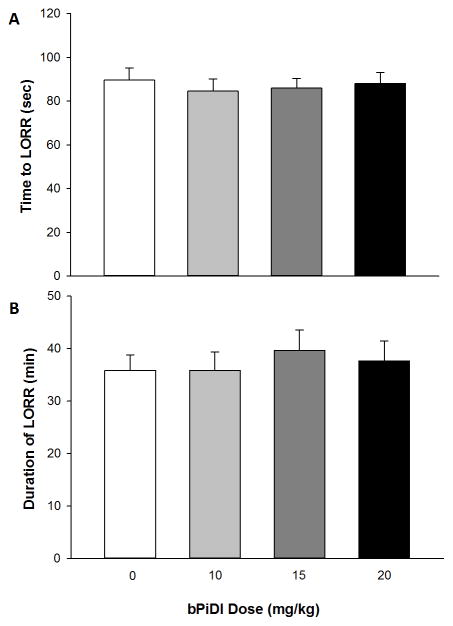
α6β2 nAChRs did not affect the sedative-hypnotic effects of ethanol in adolescent C57BL/6J mice (Fig. 4). To examine the effect of bPiDI on ethanol sedation, two dependent variables were examined: the time to LORR and LORR duration. Two separate ANOVAs with sex and dose as independent factors revealed no significant main effects or interactions for either time to LORR or duration of LORR.
Fig 4. bPiDI had no effect on ethanol’s sedative-hypnotic effects as measured by LORR.
Data (mean ± SEM) represent time to LORR (A) and duration of LORR (B). No significant main effects or interactions with sex were observed, so male and female data are combined. N = 18 – 22 animals per dose.
Metabolism
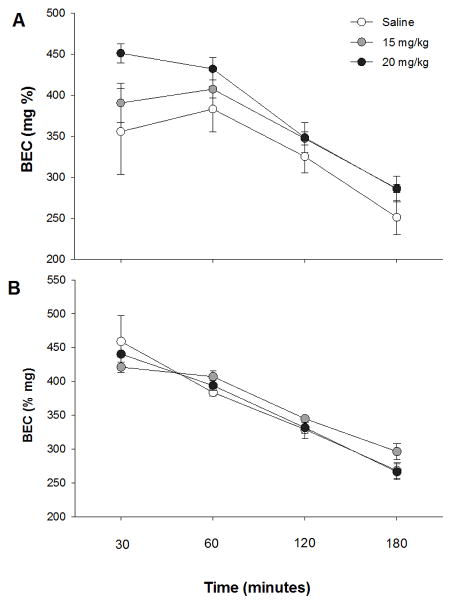
bPiDI did not significantly influence ethanol metabolism in either male or female animals (Fig 5). A repeated measures ANOVA was used to examine BEC following an acute ethanol injection (4 g/kg). Data were analyzed separately for each sex due to a significant sex X time interaction (F3, 66=4.2, p<0.01). In female mice, a significant main effect of time (F3, 33=74.7, p<0.001) was observed, but no other significant main effects or interactions were present. BEC values were not significantly different between the 30 and 60 minute time points, but differed at all other time points (p’s<0.01). Similar results were obtained in male animals, with a significant main effect of time (F3, 33=60.3, p<0.001), but no other effects. In male mice, BEC levels decreased significantly between all time points (p’s<0.05).
Fig 5. bPiDI has no effect on metabolism of an acute injection of ethanol (4 g/kg).
Data (mean ± SEM) represent blood ethanol concentrations (BEC) in female (A) and male (B) mice. N = 9 – 10 animals per dose.
Discussion
The importance of α6-containing nAChRs in ethanol behaviors has been highlighted in both human and animal work. Results from human genetics studies have found significant associations with CHRNA6 variants and alcohol phenotypes (Hoft et al., 2009; Landgren et al., 2009) and research using genetically modified animals has supported the involvement of this subunit in ethanol behaviors (Kamens et al., 2012; Powers et al., 2013). The goal of the current project was to examine the role of α6β2 nAChRs in ethanol behaviors utilizing a pharmacologic approach. We found that bPiDI transiently reduced locomotor activity, but that normal activity was restored by 30 minutes after the injection. bPiDI also reduced ethanol consumption, but the effect was not specific as it also decreased consumption of the sweet solution saccharin. bPiDI was not found to alter ethanol-induced sedation, ataxia, or metabolism.
We observed a transient decrease in locomotor activity when female mice were given bPiDI. In the ANOVA model, there was a significant main effect of sex with female mice overall more active compared to males (Fig 1). It is possible that we were able to observe a decrease in locomotor activity in the female mice because of this sex difference in locomotor activity (i.e., female mice had greater baseline to be reduced from). Additionally, it is possible that if more animals were included we may have observed a significant difference in male mice as well. The effect size of the dose X time interaction in females was d = 1.55, while in males it was d = 0.97, supporting the possibility that we may have been under-powered for this experiment. Although there was no significant effect of bPiDI dose on locomotor activity in male mice, there is an apparent depression of locomotor activity following bPiDI around the 15 minute post-bPiDI injection which may have reached significance if additional animals had been tested (Fig 1B).
Expression of the α6 subunit is limited throughout the brain, but of key importance is its expression in the substantia nigra and ventral tegmental area (Quik & McIntosh, 2006), which are regions known to modulate locomotor activity. Our data partially agree with prior literature on the role of α6 containing nAChRs in spontaneous locomotor activity. In particular, transgenic mice that overexpress a mutant hypersensitive α6 subunit exhibit more activity than wildtype animals (Cohen et al., 2012; Drenan et al., 2008, 2010). Thus, these data suggest that having a more active α6 receptors causes increased locomotor activity. Consistent with this in our data blocking receptors that contain an α6 subunit had the reverse effect – we observed decreased locomotor activity. Earlier studies revealed no effect of bPiDI on locomotor activity (Madsen et al., 2015; Srisontiyakul et al., 2016), but our study tested a higher dose. Additionally, a second factor that could explain differences in these results is that we examined adolescent animals because of high expression of the α6 subunit during this time (Azam et al., 2007). It is possible that adult mice would be less sensitive to locomotor depressant effects of bPiDI due to the reduced expression of this subunit.
In addition to observing decreased locomotor activity, we also observed that bPiDI decreased ethanol consumption. Although bPiDI decreased ethanol consumption, it also decreased saccharin consumption. Recent work in the iP rat model showed that an injection of 3 mg/kg bPiDI selectively reduced operant responding for ethanol in the absence of a difference in responding for sucrose (Srisontiyakul et al., 2016). Our work is similar in that we also show decreases in ethanol intake, but our results were not specific for ethanol. The discrepancy in results could be due to a number of experimental differences. Notably, our study used a higher bPiDI dose which may have resulted in non-specific effects. Additional differences include the species, age, sex and that the Srisontiyakul paper utilized a line of rats selected for high ethanol consumption whereas we utilized an unselected inbred strain of mice. Inconsistent results have also been found with studies of genetically modified animals. While animals lacking the α6 subunit do not differ in ethanol consumption (Guildford et al., 2016; Kamens et al., 2012), mice with a hypersensitive α6 subunit consume significantly more ethanol than wildtype animals (Powers et al., 2013). Together, these data suggest that α6β2 nicotinic acetylcholine receptors may be involved in ethanol intake but additional research is needed to clarify their role.
In order to take into account the reduction in locomotor activity we observed, we pre-treated animals 30 minutes before all behavioral tests to ensure that changes in behavior were not directly attributable to locomotor activity. This pre-treatment time is similar to other nicotinic receptor drugs that also modulate ethanol consumption and self-administration (Kamens et al., 2010a; Kuzmin, Jerlhag, Liljequist, & Engel, 2009; Steensland et al., 2007). Our main behavioral effects were seen 1 hour after the bPiDI injection (30 minute pre-treatment plus 30 DID test), this time frame is consistent with bPiDI decreasing nicotine self-administration (Madsen et al., 2015). To our knowledge there are no data on the pharmacokinetics of bPiDI. Therefore, it remains possible that bPiDI is metabolized during this time and that the effects observed are due to an active metabolite.
As mentioned above, bPiDI has been shown to reduced nicotine self-administration in both rats and mice (Madsen et al., 2015; Wooters et al., 2011). α6 expression in dopaminergic neurons in the ventral tegmental area may help explain these results (Champtiaux et al., 2002). Importantly, research has shown that both nicotine and ethanol-induced nucleus accumbens dopamine release can be blocked by an α6 nAChR antagonist (Schilaty et al., 2014), providing a mechanism by which bPiDI could decrease intake of both of these drugs. Moreover, recent work has suggested that other nicotinic receptor drugs can decrease consumption of the sweet solution sucrose and that long-term consumption of sucrose alters nAChRs in the nucleus accumbens (Shariff et al., 2016). Therefore, it is possible that α6-containing nicotinic receptors in the nucleus accumbens may modulate ethanol, nicotine, and saccharin intake.
In contrast to ethanol consumption, bPiDI had no effect on ethanol sedation or ataxia. Prior work from our laboratory utilizing α6 knockout mice (Kamens et al., 2012) partially agrees with the current data. In knockout animals, we found no difference in ethanol-induced ataxia similar to the current findings. In contrast, we did find that mice lacking the α6 gene were more sensitive to the sedative effects of ethanol. One key difference between the study with knockout animals and the current work is the age of the animals. In this study, adolescent C57BL/6J mice were tested. Adolescent mice have markedly reduced LORR durations compared to adult mice (Linsenbardt et al., 2009), but have a higher initial blood ethanol concentration when administered the same dose of the drug (Hefner & Holmes, 2007). These data suggest that sensitivity to the sedative effects of ethanol may be mediated by a different process in adolescent versus adult animals. Thus, it is possible that we may have observed different results if we had tested adult animals.
In the current work, we chose to examine the influence of α6β2 nAChRs in adolescent animals. This choice was made because of the known changes in expression of α6 mRNA across development. Specifically, α6 mRNA is highest in early adolescence in the substantia nigra and ventral tegmental area (Azam et al., 2007). It remains to be determined if similar results would be observed in adult animals. This is important because all studies of α6 genetically engineered animals have utilized adult animals (Kamens et al., 2012; Powers et al., 2013). Further work should examine the role of bPiDI in adult ethanol behaviors. Alternatively, other genetic manipulations to the subunit such as a conditional knockout may provide a greater understanding of the role of α6 nAChRs in ethanol behaviors.
The current results provide an interesting perspective in comparison to other work on genetically engineered mice. In adult genetically engineered mice, α6-containing nAChRs have been implicated in ethanol consumption and sedation (Kamens et al., 2012; Powers et al., 2013). In these mouse models the α6 subunit was altered in development, thus, it is possible that compensation may have occurred. Here we show that bPiDI reduced ethanol consumption, but also reduced consumption of the sweet solution saccharin. Further research is needed to further clarify the role of α6-containing nAChRs in ethanol behaviors.
Highlights.
α6β2 nicotinic receptors are involved in locomotor activity
α6β2 nicotinic receptors may contribute to ethanol consumption
α6β2 nicotinic receptors do not contribute to ethanol ataxia or sedation
Acknowledgments
This work was supported by the National Institutes of Health (AA019447) and the Pennsylvania State University College of Health and Human Development. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144(4):1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. https://doi.org/10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM. Deletion of the alpha7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcoholism, Clinical and Experimental Research. 2005;29(3):295–302. doi: 10.1097/01.alc.0000156116.40817.a2. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, … Changeux J-P. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22(4):1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BN, Mackey EDW, Grady SR, McKinney S, Patzlaff NE, Wageman CR, … Drenan RM. Nicotinic cholinergic mechanisms causing elevated dopamine release and abnormal locomotor behavior. Neuroscience. 2012;200:31–41. doi: 10.1016/j.neuroscience.2011.10.047. https://doi.org/10.1016/j.neuroscience.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Ponomarev I, Prescott CA, Wahlsten D. Effects of genetic and procedural variation on measurement of alcohol sensitivity in mouse inbred strains. Behavior Genetics. 2006;36(4):536–552. doi: 10.1007/s10519-006-9067-6. https://doi.org/10.1007/s10519-006-9067-6. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Yu CH, Schlumbohm JP, Cameron AJ, Wahlsten D. Genotypic differences in ethanol sensitivity in two tests of motor incoordination. Journal of Applied Physiology (Bethesda, Md: 1985) 2003;95(4):1338–1351. doi: 10.1152/japplphysiol.00132.2003. https://doi.org/10.1152/japplphysiol.00132.2003. [DOI] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Steele AD, McKinney S, Patzlaff NE, McIntosh JM, … Lester HA. Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(29):9877–9889. doi: 10.1523/JNEUROSCI.2056-10.2010. https://doi.org/10.1523/JNEUROSCI.2056-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, … Lester HA. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron. 2008;60(1):123–136. doi: 10.1016/j.neuron.2008.09.009. https://doi.org/10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol (Fayetteville, NY) 2009;43(6):443–452. doi: 10.1016/j.alcohol.2009.06.003. https://doi.org/10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Simms JA, Mill D, Yi HY, Bartlett SE. Varenicline decreases ethanol intake and increases dopamine release via neuronal nicotinic acetylcholine receptors in the nucleus accumbens. British Journal of Pharmacology. 2014 doi: 10.1111/bph.12690. https://doi.org/10.1111/bph.12690. [DOI] [PMC free article] [PubMed]
- Guildford MJ, Sacino AV, Tapper AR. Modulation of ethanol reward sensitivity by nicotinic acetylcholine receptors containing the α6 subunit. Alcohol (Fayetteville, NY) 2016;57:65–70. doi: 10.1016/j.alcohol.2016.08.006. https://doi.org/10.1016/j.alcohol.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, … Rhodes JS. Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcoholism, Clinical and Experimental Research. 2008;32(11):1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. https://doi.org/10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Haller G, Kapoor M, Budde J, Xuei X, Edenberg H, Nurnberger J, … Goate A. Rare missense variants in CHRNB3 and CHRNA3 are associated with risk of alcohol and cocaine dependence. Human Molecular Genetics. 2014;23(3):810–819. doi: 10.1093/hmg/ddt463. https://doi.org/10.1093/hmg/ddt463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. https://doi.org/10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology. 2007;191(2):311–322. doi: 10.1007/s00213-006-0646-2. https://doi.org/10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR. Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(30):10169–10176. doi: 10.1523/JNEUROSCI.2601-10.2010. https://doi.org/10.1523/JNEUROSCI.2601-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft NR, Corley RP, McQueen MB, Huizinga D, Menard S, Ehringer MA. SNPs in CHRNA6 and CHRNB3 are associated with alcohol consumption in a nationally representative sample. Genes, Brain, and Behavior. 2009;8(6):631–637. doi: 10.1111/j.1601-183X.2009.00495.x. https://doi.org/10.1111/j.1601-183X.2009.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology. 2007;192(2):207–217. doi: 10.1007/s00213-007-0711-5. https://doi.org/10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology. 2010a;208(4):613–626. doi: 10.1007/s00213-009-1759-1. https://doi.org/10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. The nicotinic acetylcholine receptor partial agonist varenicline increases the ataxic and sedative-hypnotic effects of acute ethanol administration in C57BL/6J mice. Alcoholism, Clinical and Experimental Research. 2010b;34(12):2053–2060. doi: 10.1111/j.1530-0277.2010.01301.x. https://doi.org/10.1111/j.1530-0277.2010.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Ethanol-related traits in mice selectively bred for differential sensitivity to methamphetamine-induced activation. Behavioral Neuroscience. 2006;120(6):1356–1366. doi: 10.1037/0735-7044.120.6.1356. https://doi.org/10.1037/0735-7044.120.6.1356. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Hoft NR, Cox RJ, Miyamoto JH, Ehringer MA. The α6 nicotinic acetylcholine receptor subunit influences ethanol-induced sedation. Alcohol (Fayetteville, NY) 2012;46(5):463–471. doi: 10.1016/j.alcohol.2012.03.001. https://doi.org/10.1016/j.alcohol.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, McKinnon CS, Li N, Helms ML, Belknap JK, Phillips TJ. The alpha 3 subunit gene of the nicotinic acetylcholine receptor is a candidate gene for ethanol stimulation. Genes, Brain, and Behavior. 2009;8(6):600–609. doi: 10.1111/j.1601-183X.2008.00444.x. https://doi.org/10.1111/j.1601-183X.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Phillips TJ. A role for neuronal nicotinic acetylcholine receptors in ethanol-induced stimulation, but not cocaine- or methamphetamine-induced stimulation. Psychopharmacology. 2008;196(3):377–387. doi: 10.1007/s00213-007-0969-7. https://doi.org/10.1007/s00213-007-0969-7. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Jerlhag E, Liljequist S, Engel J. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology. 2009;203(1):99–108. doi: 10.1007/s00213-008-1375-5. https://doi.org/10.1007/s00213-008-1375-5. [DOI] [PubMed] [Google Scholar]
- Landgren S, Engel JA, Andersson ME, Gonzalez-Quintela A, Campos J, Nilsson S, … Jerlhag E. Association of nAChR gene haplotypes with heavy alcohol use and body mass. Brain Research. 2009;1305(Suppl):S72–79. doi: 10.1016/j.brainres.2009.08.026. https://doi.org/10.1016/j.brainres.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Larsson A, Svensson L, Söderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol (Fayetteville, NY) 2002;28(3):157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., 2nd Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcoholism, Clinical and Experimental Research. 2009;33(3):464–476. doi: 10.1111/j.1530-0277.2008.00857.x. https://doi.org/10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhao-Shea R, McIntosh JM, Tapper AR. Nicotinic acetylcholine receptors containing the α6 subunit contribute to ethanol activation of ventral tegmental area dopaminergic neurons. Biochemical Pharmacology. 2013;86(8):1194–1200. doi: 10.1016/j.bcp.2013.06.015. https://doi.org/10.1016/j.bcp.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HB, Koghar HS, Pooters T, Massalas JS, Drago J, Lawrence AJ. Role of α4- and α6-containing nicotinic receptors in the acquisition and maintenance of nicotine self-administration. Addiction Biology. 2015;20(3):500–512. doi: 10.1111/adb.12148. https://doi.org/10.1111/adb.12148. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8. Washington (DC): National Academies Press (US); 2011. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK54050/ [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. A novel method to assess initial sensitivity and acute functional tolerance to hypnotic effects of ethanol. The Journal of Pharmacology and Experimental Therapeutics. 2002;302(1):257–263. doi: 10.1124/jpet.302.1.257. [DOI] [PubMed] [Google Scholar]
- Powers MS, Broderick HJ, Drenan RM, Chester JA. Nicotinic acetylcholine receptors containing α6 subunits contribute to alcohol reward-related behaviours. Genes, Brain, and Behavior. 2013;12(5):543–553. doi: 10.1111/gbb.12042. https://doi.org/10.1111/gbb.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, McIntosh JM. Striatal alpha6* nicotinic acetylcholine receptors: potential targets for Parkinson’s disease therapy. The Journal of Pharmacology and Experimental Therapeutics. 2006;316(2):481–489. doi: 10.1124/jpet.105.094375. https://doi.org/10.1124/jpet.105.094375. [DOI] [PubMed] [Google Scholar]
- Quik M, Perez XA, Grady SR. Role of α6 nicotinic receptors in CNS dopaminergic function: relevance to addiction and neurological disorders. Biochemical Pharmacology. 2011;82(8):873–882. doi: 10.1016/j.bcp.2011.06.001. https://doi.org/10.1016/j.bcp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. https://doi.org/10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Schilaty ND, Hedges DM, Jang EY, Folsom RJ, Yorgason JT, McIntosh JM, Steffensen SC. Acute ethanol inhibits dopamine release in the nucleus accumbensvia α6 nicotinic acetylcholine receptors. The Journal of Pharmacology and Experimental Therapeutics. 2014 doi: 10.1124/jpet.113.211490. https://doi.org/10.1124/jpet.113.211490. [DOI] [PMC free article] [PubMed]
- Shariff M, Quik M, Holgate J, Morgan M, Patkar OL, Tam V, … Bartlett SE. Neuronal Nicotinic Acetylcholine Receptor Modulators Reduce Sugar Intake. PloS One. 2016;11(3):e0150270. doi: 10.1371/journal.pone.0150270. https://doi.org/10.1371/journal.pone.0150270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisontiyakul J, Kastman HE, Krstew EV, Govitrapong P, Lawrence AJ. The Nicotinic α6-Subunit Selective Antagonist bPiDI Reduces Alcohol Self-Administration in Alcohol-Preferring Rats. Neurochemical Research. 2016;41(12):3206–3214. doi: 10.1007/s11064-016-2045-3. https://doi.org/10.1007/s11064-016-2045-3. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(30):12518–12523. doi: 10.1073/pnas.0705368104. https://doi.org/10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taslim N, Al-Rejaie S, Saeed Dar M. Attenuation of ethanol-induced ataxia by alpha(4)beta(2) nicotinic acetylcholine receptor subtype in mouse cerebellum: a functional interaction. Neuroscience. 2008;157(1):204–213. doi: 10.1016/j.neuroscience.2008.08.046. https://doi.org/10.1016/j.neuroscience.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Smith AM, Pivavarchyk M, Siripurapu KB, McIntosh JM, Zhang Z, … Dwoskin LP. bPiDI: a novel selective α6β2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse. British Journal of Pharmacology. 2011;163(2):346–357. doi: 10.1111/j.1476-5381.2011.01220.x. https://doi.org/10.1111/j.1476-5381.2011.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]