Figure 1. Mechanisms within the PDAC TME drives resistance to therapies.
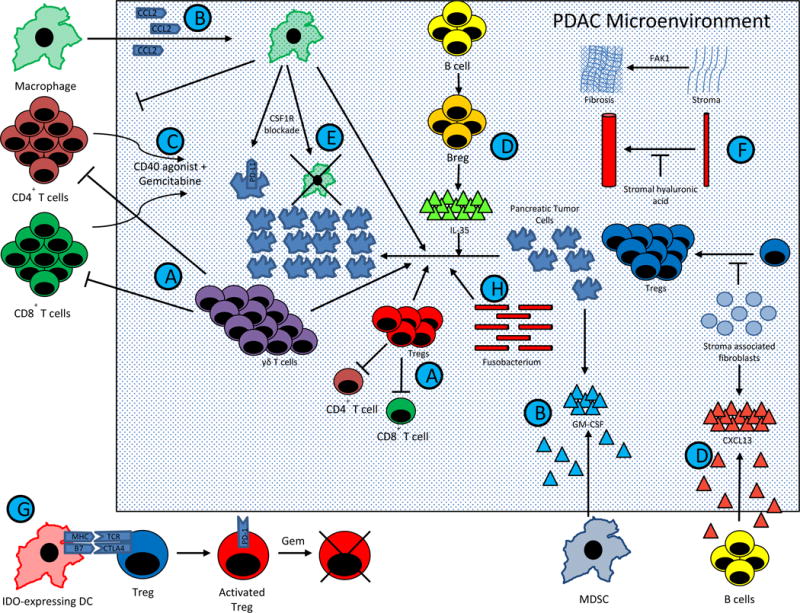
PDAC comprises of complex interactions between T cells, B cells, APCs, pancreatic tumor cells, and stromal elements. These interactions result in a profoundly immunosuppressive tumor microenvironment, and consequently single agent immunotherapy has been largely ineffective. However, emerging preclinical data has suggested that combination therapy may dramatically affect overall survival. Current trial design is being driven largely by this data. The figure summarizes major pathways in PDAC tumorigenesis that are being manipulated in clinical trials for patients with metastatic PDAC. Except for (G.), which represents in part IDO activated Tregs in TDLNs from a melanoma model (40), this figure represents data known exclusively from PDAC models.
(A.) Tregs and γδ T cells block Teff division and drive PDAC growth, while γδ T cells block T cell infiltration (47).
(B.) MDSCs and macrophages are mobilized into the TME by PDAC derived GM-CSF and CCL2, respectively. (145,183,184).
(C.) Macrophages block CD4+ T cell entry into the PDAC microenvironment. CD40 is expressed on these CD4+ T cells, and activation of the CD40 pathway concurrently with gemcitabine can drive T cell infiltration (140).
(D.) Stromal associated fibroblasts produce CXCL13, which recruits regulatory B cells into the TME. These regulatory B cells produce IL-35, which drives PDAC progression (136,185). These Bregs may be inhibited by BTK inhibitors, such as ibrutinib (137).
(E.) Tumor infiltrating macrophages stimulate PDAC progression. Blockade of the CSF1 receptor expressed by macrophages can lead to macrophage depletion, CTLA-4 upregulation on CD8+ T cells, and PD-L1 upregulation on pancreatic tumor cells (146,147).
(F.) Stromal elements create a physical barrier to immune infiltration and therapeutic agents. Stromal fibroblasts block Treg accumulation and PDAC progression (62), but targeting other stromal elements have achieved encouraging results. Stromal hyaluronic acid deposition results in decreased vascular patency (72,73), and FAK1 drives stromal fibrosis (68). Inhibition of either target has led to decreased PDAC progression when combined with chemotherapy in preclinical models.
(G.) IDO induction in DCs by tumors activate Tregs via MHC and CTLA4 pathways (40,131). In phase II studies, gemcitabine based therapy synergizes with IDO inhibition to improve response rates in PDAC (133), possibly via transient depletion of Tregs (39). This provides an immune system reset, allowing for chemotherapy-mediated elimination of previously activated Tregs, followed by indoximod mediated inhibition of subsequent Treg activation.
(H.) Recent evidence suggests the Fusobacterium found within the PDAC microenvironment drives PDAC progression, but the mechanism of this is unknown (91).
