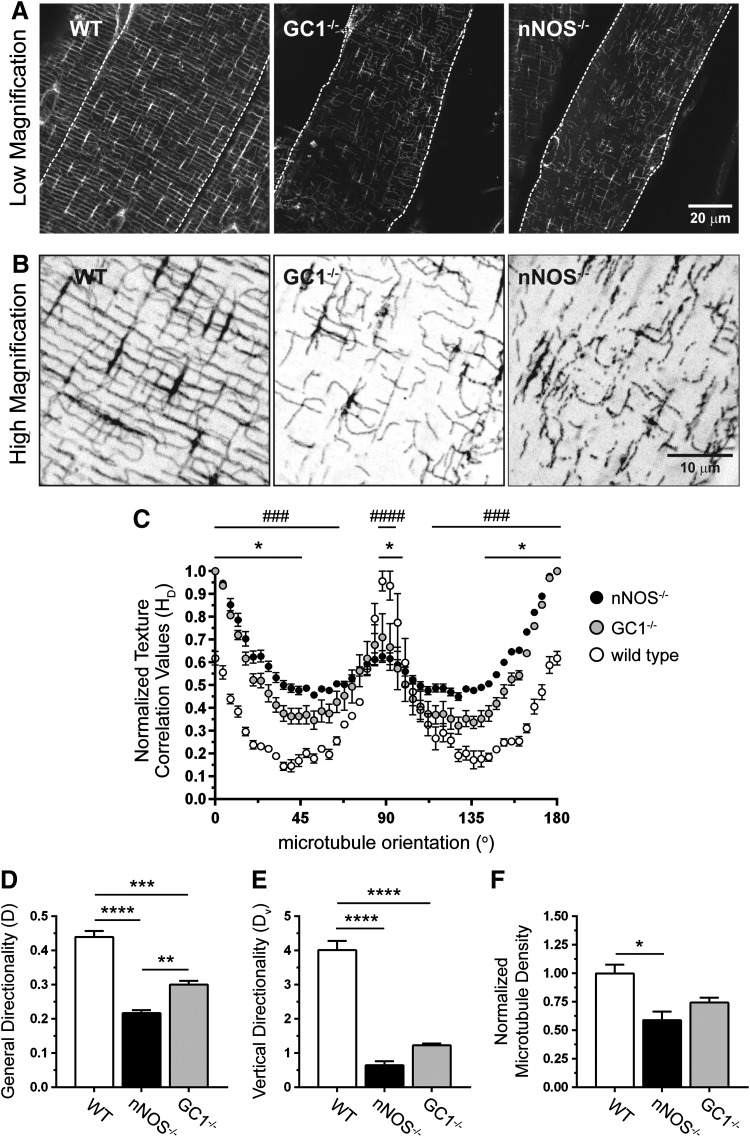
FIG. 5.
nNOS regulates subsarcolemmal microtubule lattice organization through a GC1-cGMP-dependent mechanism. (A) Representative low-magnification confocal micrographs of the subsarcolemmal microtubule cytoskeleton immunolabeled with FITC-conjugated anti-α-tubulin antibody in TA myofibers from WT, GC1−/−, and nNOS−/− mice. (B) High-magnification versions of regions within the confocal micrographs in (A). (C) Histogram of subsarcolemmal microtubule general directionality in TA myofibers from WT, GC1−/−, and nNOS−/− mice. (D) Subsarcolemmal general microtubule directionality scores in WT, GC1−/−, and nNOS−/− TA muscles. (E) Quantitation of vertical (microtubules orthogonal to the myofiber long axis) microtubule directionality (Dv) in WT, GC1−/−, and nNOS−/− TA myofibers. (F) Quantitation of subsarcolemmal microtubule densities in TA myofibers from WT, GC1−/−, and nNOS−/− mice. (A–F), n = 3–4 for all groups. For (C), ###p < 0.001; ####p < 0.0001 for WT versus nNOS−/−; and *p < 0.05 for WT versus GC−/− from regular two-way ANOVA using orientation and genotype as variables with Tukey's multiple comparison test. For (D–F), *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 by one-way ANOVA with Tukey's multiple comparison test.