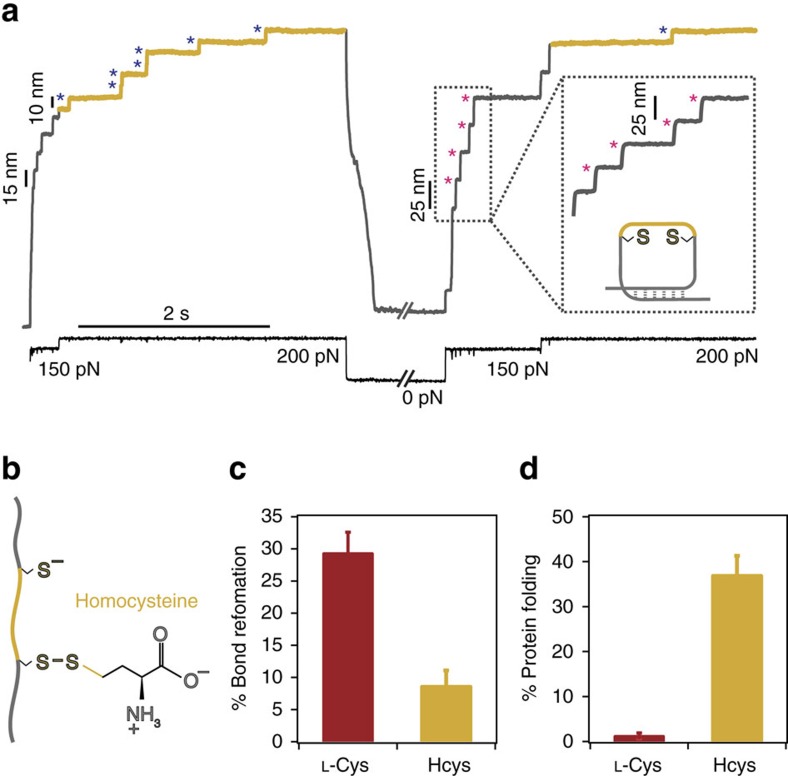
Figure 2. DL-homocysteine prevents successful non-enzymatic oxidative folding.
(a) The oxidative folding trajectory in the presence of homocysteine (111 mM, pH=7.6) exhibits a test pulse that is composed of steps of ∼25 nm (marking the refolding of a reduced protein form, pink asterisks) followed by a vanishingly small population of ∼10 nm steps, fingerprinting disulfide bond reformation (blue asterisks). (b) The asymmetric mixed disulfide between a native cysteine and the attacking homocysteine, which only differs from L-cysteine in the presence of a methylene group (yellow) next to the nucleophilic thiol plays a crucial role in the outcome of the folding reaction. (c) While the percentage of disulfide bond reformation in the presence of homocysteine (yellow) is negligible compared to that of L-cysteine (red), (d) the situation is reversed for the population of folded and reduced proteins (∼25 nm steps), which is largely predominant for homocysteine (yellow) when compared to the residual fraction measured for L-cysteine (red) (error bar±s.d.).