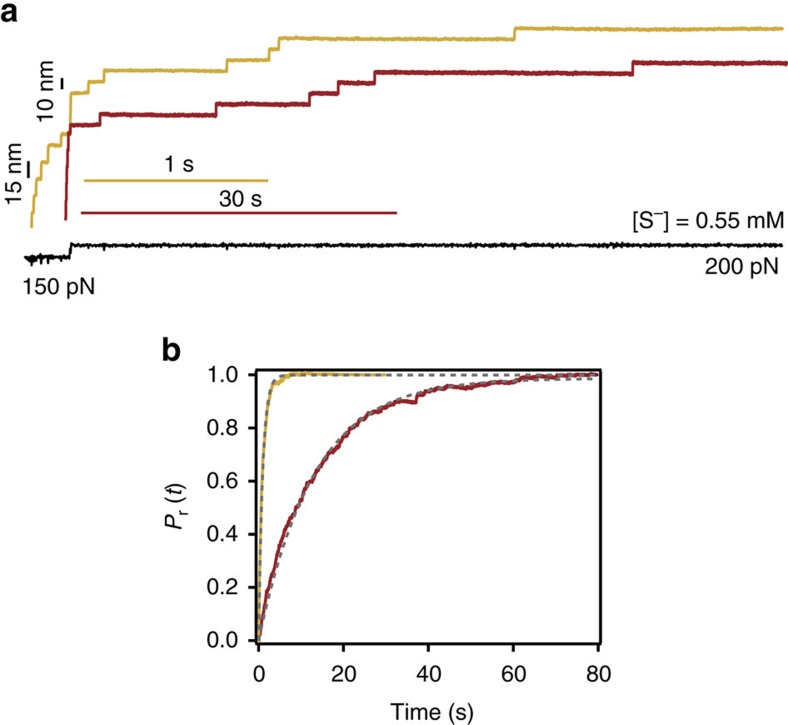
Figure 5. The kinetics of disulfide reduction is largely accelerated by homocysteine due to its high nucleophilic power.
(a) A two-pulse force protocol measures the kinetics of disulfide reduction when the protein is exposed to the same concentration of active deprotonated species of L-cysteine (red) and homocysteine (yellow). The time-course of disulfide bond reduction is dramatically longer for L-cysteine as illustrated by the drastically different scale bars (b) The rate of disulfide bond reduction at F=200 pN is ∼14-fold faster for homocysteine (r=1.11 s−1) than for L-Cys (r=0.08 s−1) under the same experimental conditions, demonstrating that homocysteine is a better nucleophile than L-cysteine.