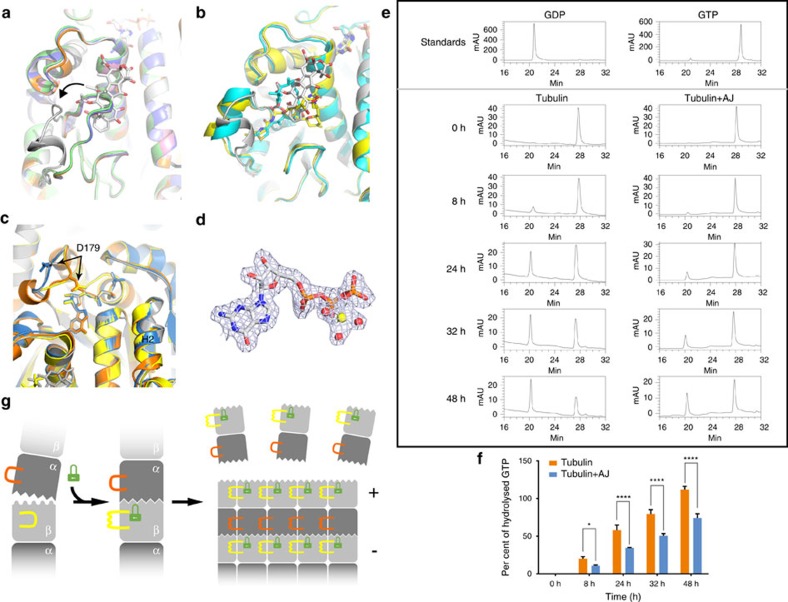
Figure 4. The microtubule-stabilizing mechanism of AJ.
(a) Superposition of tubulin–AJ (grey) and taxane-site-free tubulin structures (5CA0: pink; 5CA1: orange; 5C8Y: blue; 5CB4: green) shows the closed-to-open conformational change of the β M-loop. (b) Superposition of tubulin–MSA structures (AJ: grey; Zampa: yellow; EpoA: cyan) shows the helix conformation of the β M-loop. (c) Comparison of E-site conformations. The β-subunits of AJ-bound tubulin (grey), GTP-preferred status tubulin (3RYF: blue), Zampa-bound tubulin (orange) and EpoA-bound tubulin (yellow). (d) Electron density of GTP at the E-site in tubulin–AJ complex, with a magnesium ion (yellow) and three water molecules (red). (e) Reverse-phase HPLC (RP-HPLC) assay shows that AJ binding significantly delayed E-site GTP hydrolysis. (f) Quantification of the hydrolysis of the E-site GTP. The area of GDP and GTP peaks in e are calculated. Values are means±s.d. (n=3). *P<0.05; ****P<0.0001. (g) Proposed microtubule-stabilizing mechanism of AJ. Binding of AJ (green lock) to the taxane-site induces the β M-loop (yellow) to undergo a closed-to-open and loop-to-helix conformational change, and locks the E-site into a GTP-preferred conformation (sawtooth). The GDP-status conformation is shown as wave.