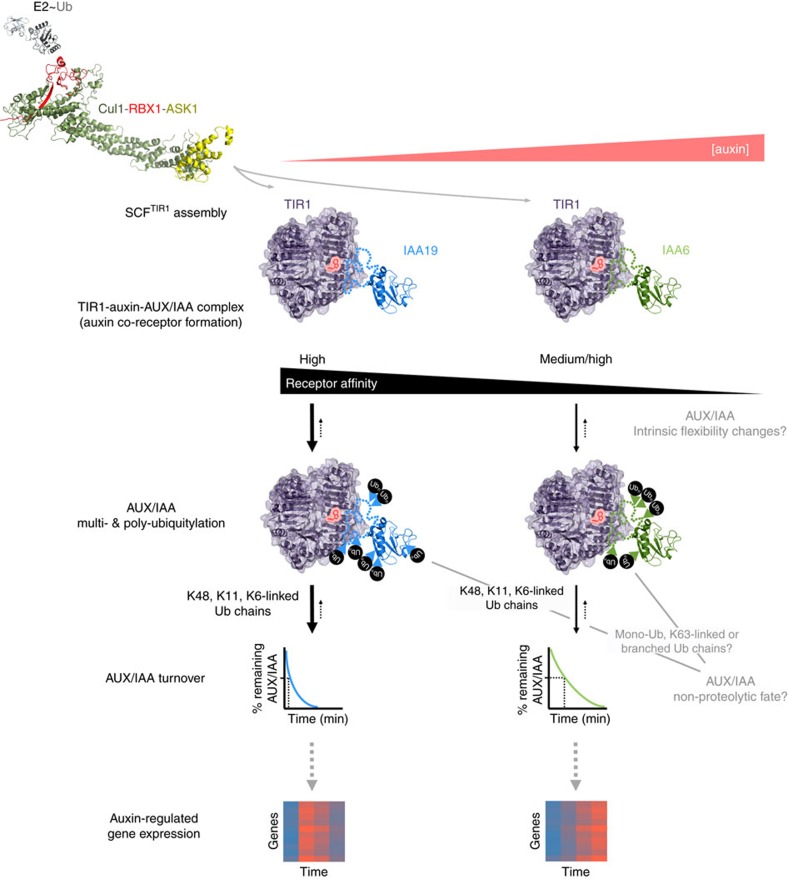
Figure 5. Simplified model of auxin sensing by SCFTIR1-IAA6 and SCFTIR1-IAA19 co-receptor complexes.
A Cullin-RING E3 ligase80 from the SCF-type is formed when TIR1 or its paralogs AFB1-5 interchangeably assemble with the adaptor protein ASK1. SCFTIR1 interacts with both E2∼Ub and IAA6 or IAA19 degradation targets in response to intracellular auxin levels. TIR1 recruits IAA19 at low nanomolar concentrations of IAA and forms a high affinity co-receptor complex, while TIR1-IAA6 displays only a medium IAA affinity. Co-receptor complex dissociation is possible but unfavored in the presence of auxin (reverse dotted arrows). A degron and intrinsically disordered regions (unstructured dotted line) most likely fit on top of auxin in the TIR1-auxin-binding groove. It is currently unknown whether the two-pronged PB1-like IAA6 and IAA19 homo- and heterodimerization domains III-IV (folded structure) directly contribute to auxin binding. IAA binding affinities of TIR1-IAA6 and TIR1-IAA19 complexes yield differential Ub-conjugation at different sites. Lysines (K) along the IAA19 structure probably become ubiquitylated with Lys48-, Lys6-, Lys11-chain linkage types offering multiple ubiquitylation signatures for efficient and rapid degradation by the 26S proteasome. Putatively IAA6 ubiquitylation on lysine residues might be less efficient, leading to a comparably slower IAA6 turnover. Other residues in flexible and/or intrinsically disordered regions of IAA6 and IAA19 eventually become ubiquitylated in vivo. Since the outcome of AUX/IAA ubiquitylation depends on the distinct types of ubiquitin topologies, K63-linked ubiquitin chains, monoubiquitylation or mixed chains on IAA6 and IAA19 could affect their function and have a non-proteolytic role. Conceivably, AUX/IAA ubiquitylation can be counteracted by the activity of deubiquitylases (reverse dotted arrows). AUX/IAA ubiquitylation, particularly initial rounds, might trigger temporal- and auxin- dependent SCFTIR1-AUX/IAA binding specificity variations through intrinsic flexibility changes. IAA19 has a very short half-life, its ohnologue IAA6, although also unstable, exhibits longer half-life, which is a reflection of their differential affinity for auxin when in TIR1-containing co-receptor complexes. Consequently, IAA6- and IAA19-dependent specific transcriptional outputs, in different tissues and in response to different auxin concentrations, are likely impacted by AUX/IAA processing.