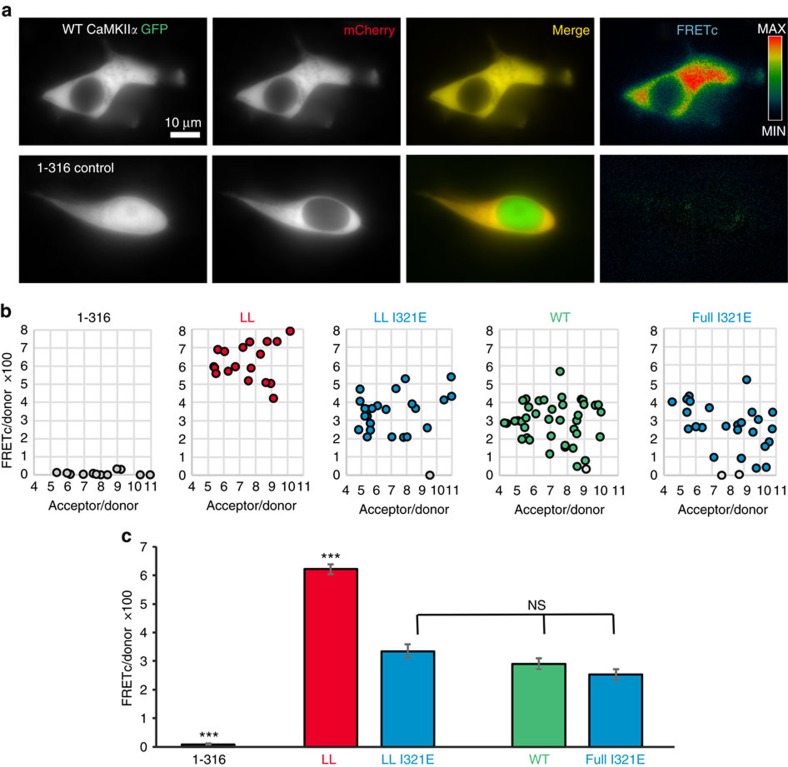
Figure 6. A FRET-based assay indicated a compact conformation within cells only for linker-less but not for full-length CaMKIIα wild type.
(a) Co-expression of CaMKIIα labelled with mCherry or mGFP (as FRET donor or acceptor) N-terminal of the kinase domain resulted in a significant FRET signal in HEK cells (FRETc; corrected for fluorescence bleed-through). Deletion of the hub domain in the GFP-labelled CaMKII (1–316; n=12 cells) abolished the FRET signal almost completely. Scale bar=10 μm. (b) FRETc was normalized by expression levels of the FRET donor (FRETc/donor) and plotted as function of the acceptor/donor ratio (within a range of 4–11 fold acceptor access). The few cells that showed complete FRET failure (grey) remained included in the analysis. (c) The linker-less (LL) CaMKIIα mutant (n=17 cells) showed significantly higher FRET than all other constructs (***P<0.001; ANOVA with Tukeys post-hoc analysis). FRET of full-length CaMKIIα wild type (WT; n=38), of its I321E mutant (full I321E; n=26) that is incompetent for the compact conformation, and of a linker-less I321E mutant (LL I321E; n=22) were indistinguishable (NS: P>0.05). Error bars represent the s.e.m.