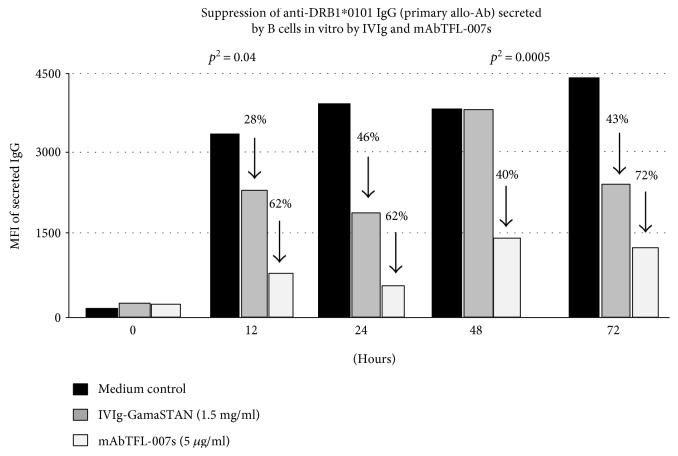
Figure 2.
HLA molecular typing of an alloimmunized woman's family showed that the first child shared the father's HLA-II type (DRB1∗0101 and DQA1∗0101/DQB1∗0501). Consequently, the mother had high levels of allo-Abs (based on mean fluorescent intensity observed with Luminex single-antigen bead assay) against both the DRB and DQ alleles, even 23 years after alloimmunization, indicating the presence of long lived Bmem cells. The allo-Abs with affinity for husband's HLA class II [primary alleles] are designated as “primary allo-Abs.” The sera also contained “secondary allo-Abs” reacting to DRB1∗0102, DRB1∗0404, DRB1∗0405, DRB1∗1402, and DRB1∗0401 (possibly those cross-reactive to the primary alleles). The B cells were isolated from the fresh peripheral blood of the mother. Using Ficoll-Paque PLUS, the peripheral blood mononuclear cells (PBMC) were isolated. The B cells (resting) were isolated from the PBMC by positive selection using CD19 Pan B Dynabeads® magnetic beads. B cells were detached by DETACHaBEAD® CD19. Purified human B cells were >95% CD19+, as determined by flow cytometry analysis. Purified B cells were plated at 0.2 × 106/200 μl/well in a sterile 96-well, round-bottom plate. B cells were cultured in Iscove's modified Dulbecco's medium, containing HEPES, L-glutamine, and sodium pyruvate, supplemented with 10% AB human serum, 5 μg/ml recombinant human (rh) insulin, 50 μg/ml rh transferring, 25 μg/ml gentamicin, and 50 μM 2-mercaptoethanol (2-ME). The resting B cells were activated with 25 ng/ml rh IL-2, 100 ng/ml rh IL-4, 100 ng/ml rh IL-6, 50 ng/ml rh IL-10, 50 ng/ml rh IL-21, and 1 μg/ml human CD40 Ab. On day 7 of the culture, 10 μl of culture supernatant from each well was analyzed for the presence of anti-HLA class II IgG allo-Abs. Cells from the wells that contained the HLA Abs were further harvested, washed three times, seeded into 4 wells, and activated as above. On days 8 and 9, the culture supernatants were tested for the secretion of allo-HLA Abs. The cells were pooled, washed (3×), and aliquoted into 3 wells: with medium alone; with GamaSTAN IVIg at 1/100 dilution, 1.5 mg/ml; and with mAb TFL-007s at 1/100 dilution containing 5 μg/ml. The cells were maintained in culture without any cytokine activators or anti-CD40 Ab for an additional 3 days, and 10 μl of culture supernatants from each well was analyzed for HLA allo-Abs at hours 0, 12, 24, 48, and 72. The figure shows the paired sample analysis of the triplicates at different hours. The paired sample two-tailed t-test was used to compare the results obtained at the stated hours with IVIg and TFL-007s against those for the control wells. The paired sample two-tailed t-test was carried out for IVIg and TFL-007s separately. Combined mean of the triplicate values obtained at 12 to 72 hrs for IVIg and TFL-007s was against the pooled values for the medium only of control wells (details in [48]).