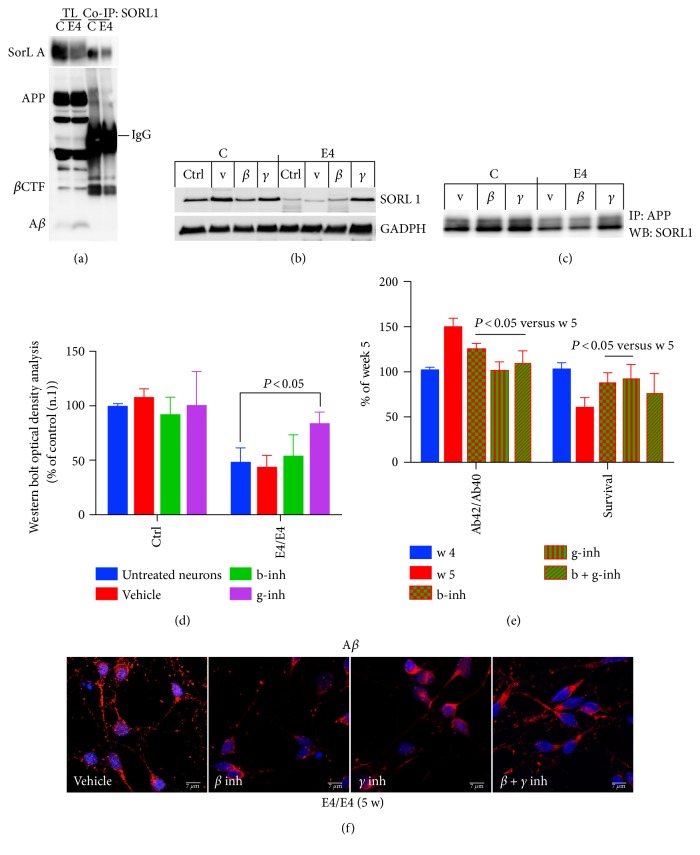
Figure 3.
SORL1 binding to APP is decreased in E4/E4 neurons. (a) Protein samples from control (C) and E4/E4 (E4) neurons, after five weeks in culture, were immunoprecipitated with mouse anti-SORL1 antibody (co-IP SORL1) and analyzed with rabbit anti-APP. TL, total lysate; Co-IP, immunoprecipitate. (b) WB analysis of control (C) and E4/E4 (E4) neurons after five weeks in culture (Ctrl, untreated neurons) with vehicle (V, DMSO 0.01%) β– (b-inh, 250 μM), and γ– (g-inh, 50 nM) secretase inhibitors. (c) The same samples were immunoprecipitated with anti-APP antibody and analyzed with anti-SORL1 by WB. Densitometric analysis (OD) is reported in (d) n = 4; one-way ANOVA with post hoc Tukey's test. P < 0.05 versus untreated E4/E4 neurons. (e) To counteract Aβ42 production, neurons were exposed to β– (b-inh, 250 nM), γ– (g-inh, 50 nM), and β + γ (b + g-inh) secretase inhibitors after four weeks of plating (blue histogram), and Aβ42 levels were assessed one week later, five weeks (red histograms) by ELISA. (f) Confocal microscopy analysis of E4/E4 neurons incubated with or without secretase inhibitors and stained with an anti-Aβ antibody. Nuclei are marked in blue. Note that the anti-Aβ antibody also detects full length APP. Pictures are representative of four experiments (n = 4) performed in triplicate. Scale bar: 7 μm. Media with or without inhibitors were refreshed every two days. The level of Aβ42 (expressed as the ratio of Aβ42/40) and the extent of intact NeuN-positive nuclei (survival) are reported in (e). Each data point is the mean ± SEM of triplicate determinations of three independent experiments (n = 3) and is expressed as the percentage of values from E4/E4 neurons after 4 weeks of plating.