Abstract
The maintenance of tissue homeostasis is indispensable for health. In particular, removal of toxic compounds from cells and organs is a vital process for the organism. The lymphatic vasculature works in order to ensure the efficient removal of tissue waste. Forbidden over the last decade when more attention was paid to the blood vasculature, studies on the lymphatic vasculature have gained momentum during the last couple of years. The lymphatic vasculature naturally runs parallel to the blood vasculature and their synergistic work is critical for maintaining tissue homeostasis. Diminished lymphatic function results in accumulation of body fluids in tissues and gives rise to edema. Recently it became obvious that immune cells including myeloid cells and lymphocytes are able to interact with and control the development and function of the lymphatic vasculature. In this review, we will focus on the interaction between myeloid cells, including macrophages, monocytes and dendritic cells, with lymphatic vessels.
Keywords: Myeloid cells, Macrophages, Dendritic cells, Lymphatic system
Introduction
1. The lymphatic vasculature
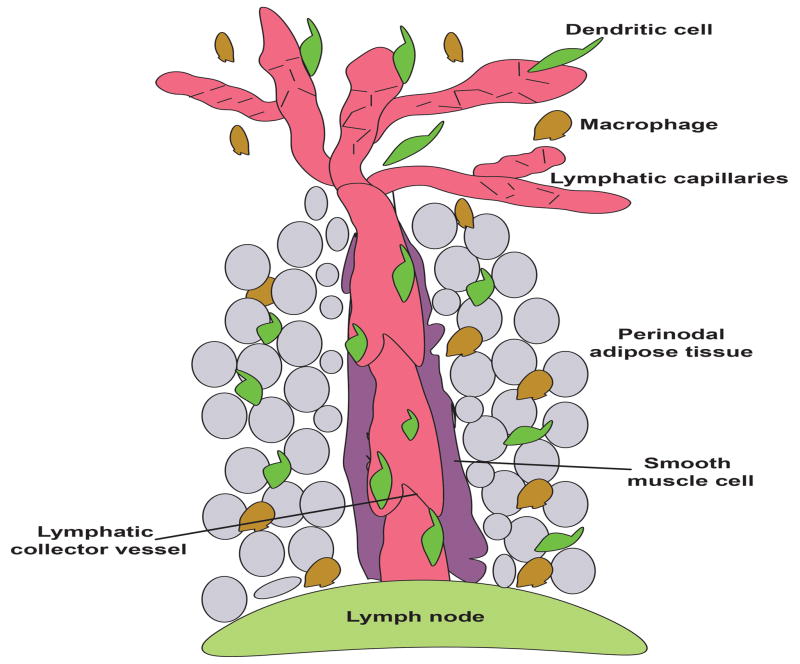
The lymphatic system runs parallel to the blood circulatory system. The expression of specific markers such as prospero homeobox 1 (Prox1), vascular endothelial growth factor receptor 3 (Vegfr3) and lymphatic vessel endothelial hyaluronan receptor 1 (Lyve1) allows for the identification of the lymphatic vasculature (6, 28, 72). Additionally, the membrane glycoprotein podoplanin is also expressed on lymphatic endothelial cells but is absent on blood endothelial cells (9). The lymphatic network begins in the periphery with vessels characterized by a thin wall and a large luminal space (for review (54)). These vessels are the so-called blind-ended lymphatic capillaries with diameter broadly varying from 10–80 μm, and their main function is the absorption of molecules from the surrounding tissue. The lymphatic capillaries do not possess independent contractile tone and rely on the movement from the extracellular matrix and cell-cell junction, in addition to the action of downstream contractile lymphatics, in order to maintain efficiently their function. Immune cells, almost exclusively CD4+ T cells and dendritic cells (DC), enter the lymphatic vasculature at the level of these capillaries. The basement membrane around the lymphatic capillaries is sparse, which facilitates the entry of DC because it limits physical barriers to cross (51). Indeed, DC entry into lymphatic capillaries under steady-state conditions does not require integrins (36). Blind-ended capillaries form a vast network and later coalesce into larger vessels known as lymphatic collectors. These larger conduits (50–200 μm in diameter) are composed of a single layer of lymphatic endothelial cells surrounded by spider-shaped smooth muscle cells. The presence of smooth muscle cells and local innervation confers autonomous contractile activity to lymphatic collectors. To maintain lymph flow, lymphatic valves are present along these vessels, designed to promote unidirectional flow. The distance between two consecutive lymphatic valves is defined as a lymphangion (Figure 1). Sometimes only a single afferent lymphatic collecting vessel enters the lymph node, where some lymph node receive many afferent inputs. Typically, there is a sole efferent lymphatic vessel that exit from the lymph node.
Figure 1.
Lymphatic collector vessels are inevitably surrounded by adipocytes. This adipose tissue contains multiple immune cells including among others dendritic cells and macrophages. Dendritic cells enter in lymphatic capillaries and migrate to local draining lymph node via the afferent lymphatic collector vessel.
Lymphatic collectors were for a long time considered as simple highways allowing the transit of molecules and immune cells from peripheral tissues to the local draining lymph node. This view started to evolve recently but additional research on lymphatic collecting vessels remains needed. For instance, lymph nodes and lymphatic collecting vessels, in contrast to lymphatic capillaries, are inevitably covered by adipose tissue, named perinodal adipose tissue (PAT). The role of this adipose tissue depot remains unknown. It is interesting that adipose depots proximal to lymphatic collecting vessels and lymph nodes are beige, with the capacity to contain a preponderance of white or brown type of adipocytes, that store fat or generate heat respectively (Figure 1 and (7)).
2. Interaction between macrophages and lymphatic vessels
Macrophages are key players involved in the maintenance of tissue homeostasis, ingestion of apoptotic cells, and defense against infection (for review,(2, 43, 68). Initially described by Elie Metchnikoff in the end of 19th century for their role in pathogen elimination, macrophages are still a subject of intensive research. Recently, the Immgen project (Immgen.org) and BioGPS gene-annotation portal (biogps.gnf.org) greatly contributed to the expansion of our knowledge about the transcriptional signature of these cells. Indeed, thanks to the genomic libraries generated by Immgen and BioGPS, it became clear that macrophages residing in different organs possess a unique transcriptional footprint (19). This led to the identification of key transcription factors specific for each population of tissue macrophages. For example, GATA6 (GATA Binding Protein 6) was predicted to be the master regulator of peritoneal macrophages, and this was further confirmed by 3 independent studies (18, 49, 56). Red pulp splenic macrophages are dependent on the transcription factor SpiC (32), which is highly specific for this population. Thanks to the Immgen project, new and more specific strategies were developed, allowing the separation of macrophages from dendritic cells, two populations of myeloid cells sharing a multitude of overlapping cell surface markers (19, 45). Recently, it became clear that immune cells not only use the lymphatic vasculature for their transit but also actively interact with the vessels and influence their development and functions. In this review we will focus on the interaction between myeloid cells, and in particular macrophages and DCs, with the lymphatic endothelium.
2.1 Lessons learned from genetic models
Genetic models were generated to investigate the role of tissue resident macrophages during steady state and disease. The first, and probably most studied, mouse model lacking tissue resident macrophages is the op/op mouse. The use of osteopetrotic op/op mice (11), bearing a null mutation in the Csf-1 gene encoding M-CSF and thus lacking multiple populations of tissue resident macrophages (74), provides data suggesting that there is an important interactive relationship between the lymphatic vasculature and M-CSF-dependent cells of the myeloid lineage (34). Interestingly, M-CSF deficiency does not affect the maintenance of the lymphatic vessels in adults but rather influences the developmental establishment of the lymphatic network, in locations other than the diaphragm, where loss of macrophages increased lymphatics (47). That is, op/op mice lack resident diaphragm macrophages and this is correlated with an increased density of the local lymphatic network. Thus, one may argue that the relationship between macrophages and lymphatics is tissue specific. Macrophages residing in different tissues have diverse origins, in addition to transcriptional signatures (13, 19, 21, 37, 49). Thus, it seems logical that these cells also have selective and differential ability to produce factors involved in the control of the lymphatic system.
Additionally, a second layer of complexity comes from the observation that lymphatics in different organs might require specific growth factors for their development and maintenance (52). Recently, a second ligand for M-CSF1R (Colony stimulating factor 1 receptor) was identified. This cytokine is named interleukin-34 (IL-34) and plays a critical role for the maintenance of skin Langerhans cells and microglia in brain (22, 70). Langerhans cells are almost completely absent in IL-34-deficient mice and microglia are half reduced. A recent report shows the presence of lymphatic vessels in the meninges of the brain (40). However, it remains unknown whether microglia and brain lymphatic endothelial cells interact. To address this question, IL-34 deficient mice might be informative. Interestingly, mice deficient for the transcription factor PU.1 and lacking cells of the myeloid lineage have lymphatic hyperplasia in the skin (20). This surprising observation has been confirmed in two different genetic backgrounds bearing PU.1 deficiency, demonstrating that the different genetic background of the mouse strain does not account predominantly for the phenotype. Additionally, Csf1r−/− mice also display increased lymphatic vasculature in skin (20). Lymphatic endothelial cells are highly proliferative in PU.1−/− mice, providing a potential explanation for the increased density of the lymphatic vasculature. However, how and why macrophages might quell this proliferation is unknown.
Monocytes use the chemokine receptor CCR2 to egress from the bone marrow and to enter peripheral tissues from the blood circulation. However, most tissue resident macrophages are not decreased in CCR2−/− mice, though some specific populations of macrophages derived from monocytes are (25, 31, 35, 65). Interestingly, mice deficient for CCR2 are characterized by decreased lymphatic vasculature density (38). Additionally, absence of the decoy receptor ACKR2, binding the CCR2 ligand CCL2, leads to augmented lymphatic network in the skin (38). These data suggests that monocytes and/or monocyte-derived macrophages in the skin promote the development of the lymphatic vasculature. Using various genetic models, the data taken as a whole are consistent with a model in which tissue resident macrophages repress the growth of the lymphatic network whereas monocyte-derived macrophages, such as inflammatory macrophages, promote lymphatic expansion.
2.2 Macrophage interaction with the lymphatic vasculature during inflammation
In the following paragraphs, we will focus our attention on the interaction between macrophage and lymphatic vessels in the context of inflammation, and we will illustrate the significance of this dynamic interaction through several examples.
High salt diet
The maintenance of tissue homeostasis is critical for the organism. The use of high salt diet in mice surprisingly revealed that sodium homeostasis could be accompanied by the accumulation of this ion in the skin. This storage was associated with increased lymphatic density in the skin compared to animals that received control diet. Macrophages were found to be key players that regulated lymphatic vessel hyperplasia during high salt diet (HSD)(41). At the cellular level, the osmoprotective transcription factor tonicity-responsive enhancer-binding protein (TonEBP) becomes activated and induces the production and release of large amounts of pro-angiogenic factors such as VEGF-C (73). Although TonEBP expression is not solely limited to myeloid cells, macrophage-selective deletion of TonEBP blunts VEGF-C expression and abolishes the lymphatic hyperplasia induced by high salt diet. Skin macrophages are heterogeneous population containing multiple subsets (65), and it is also possible that lymph node dendritic cells are the central culprit of the TonEPB deletion (48). It will be of great interest to define the specific contribution of each of these subsets of myeloid cells and their ability to secrete pro-lymphangiogenic factors.
Bacterial Infection
Inflammatory scenarios including bacterial infections are associated with increased lymphatic vessel density. Cutaneous infection with Leishmania major induces lymphatic remodeling in skin. Inhibition of VEGFR-2 signaling aggravates disease pathology by affecting lymphatic endothelial cells but not blood endothelial cells (71). Peritoneal administration of lipopolysaccharide (LPS) extracted from Gram− bacteria induces increased density of the lymphatic vasculature (30). Interestingly, LPS-induced inflammation induces a close physical association between lymphatic endothelial cells and CD11b+ macrophages. These macrophages secrete VEGF-C and -D among other angiogenic factors. Their depletion with clodronate-loaded liposomes partially abolishes the density of the lymphatic vasculature. The same observation is also reported in skin (29). Skin inflammation induced by administration of compounds extracted from Gram− (LPS) or Gram+ (lipoteichoic acid, LTA) bacteria induced lymphangiogenesis. This lymphangiogenesis requires the presence and activation of macrophages residing in the skin. However, it remains currently unknown whether tissue resident or monocyte-derived macrophages are involved in this process. In a mouse model of airway infection with Mycoplasma pulmonis, a spectacular remodeling of the lymphatic vasculature was reported (5). This was associated with production of VEGF-C by F4/80+ cells. However, whether these cells are in fact macrophages and their relative contribution to inflammatory lymphangiogenesis remains to be elucidated. Interestingly, in a mouse model of virus infection with Herpes simplex virus 1, the inflammatory lymphangiogenesis is not affected by macrophage depletion with clodronate-loaded liposomes (75).
Cancer
Cancer is often associated with an inflammatory state and secretion of pro-lymphangiogenic factors, and therefore many cancers are characterized by intratumoral or peritumoral lymphangiogenesis. Macrophages support inflammatory lymphangiogenesis, as their depletion inhibits generation of new vessels (12). Some cancers use lymphatic vessels to exit the primary cancer and give rise to metastasis in the local lymph node or distant tissues (53, 55). Lymphatic vessel density correlates with local draining lymph nodes and organ metastasis (53, 55). In a mouse model of overexpression of VEGF-C, characterized by augmented lymphatic vessel density, lymphatic metastasis is increased and in patients high plasma concentration of VEGF-C correlates with increased lymph node metastasis (42, 61, 77). Tumor-associated macrophages, through the production of lymphangiogenic factors VEGF-C and -D, stimulate the expansion of lymphatic vessels. The importance of this mechanism was demonstrated when depletion of macrophages with clodronate-loaded liposomes abolished cancer-induced lymphangiogenesis in multiple tumor models (14, 76). Taking into account that clodronate-loaded liposome induces macrophage loss wherever it acts, it would be of great interest to revisit this question using newly described tools that allow for tissue-selective depletion of resident macrophages without affecting other cells or organs. For example, Clec4f is a Kupffer cell specific gene and Clec4f-DTR mice allow for transient and specific depletion of liver-resident macrophages (60). Tissue-specific deletion of liver macrophages will provide an exciting model to study the interaction between macrophages and lymphatic endothelial cells under homeostatic conditions or during diseases such as cancer in the liver.
3. Dendritic cells and lymphatic vessels
Dendritic cells (DCs) were discovered in the early 1970s by Ralph Steinman and his colleagues (62–64). They were traditionally known for their ability to capture antigens in peripheral tissues and then, following maturation and migration to local draining lymph nodes (LNs). The unique structural organization of the LN facilitates the encounter of antigen-bearing DC and T cells and this leads to the activation of T cells and the development of an organized and efficient immune response. Two types of dendritic cells have been previously described and respectively named conventional DC (cDC) and plasmacytoid DC (pDC)(for review (44, 46)). Furthermore, according to the expression of the cell surface markers CD11b and CD103 the population of cDC has been shown to contain two major subsets in peripheral tissues. The transcriptional master regulators for each of these populations were identified and the transcription factor BATF3 (Basic Leucine Zipper ATF-Like Transcription Factor 3) is indispensable for the development of CD103+ DC (26). Mice deficient for BATF3 completely lack the subset of CD103+ DCs but exogenous IL-12 administration restores, at least partially, the normal number of these cells (66). The second major subset of DC expresses high levels of the integrin CD11b but lacks CD103. The transcription factor IRF4 (Interferon Regulatory Factor 4) was predicted to control the development of these cells but selective deletion of IRF4 in DC resulted in only partial deletion of CD11b+ DC in a tissue-specific manner (50, 58, 67). Indeed, in CD11ccre x IRF4fl/fl pulmonary and mediastinal lymph node CD11b+ DCs are reduced in comparison to control mice (58). The remaining pulmonary CD11b+ DCs have an apoptotic appearance suggesting a pro-survival role of IRF4 in pulmonary DCs. Interestingly, CD11b+ DCs in liver and dermis are unaffected in CD11ccre x IRF4fl/fl mice.
3.1 Interaction of DCs with lymphatic capillaries
Immature DCs are present in peripheral tissues. Upon encounter with a foreign antigen they engage in a process of maturation characterized by increased expression of the major histocompatibility complex class II (MHC II), co-stimulatory molecules such as CD40, CD80 and CD86 and the chemokine receptor CCR7. The ultimate purpose of this biological process is to ensure efficient migration to the local draining lymph node and the initiation of a T cell response. The chemokine receptor CCR7 plays a critical role in the migration of DC from the periphery to lymph nodes through the lymphatic vasculature. This chemokine receptor was initially identified in a series of papers between1993–1995 (8, 10, 59) and then knocked out in mice in 1999 by Martin Lipp, Reinhold Förster, and colleagues (16). At the same time, it became evident that the interaction between CCR7 and its two ligands (CCL19 and CCL21) governs the migration of DCs and T cells from peripheral tissues to the lymph node (23). Indeed, CCR7-deficient DCs completely fail to migrate to LNs (16). Lymphatic endothelial cells constitutively express the chemokine CCL21 (24). In the mouse model, duplication of the CCL21 gene is responsible for the appearance of two genes encoding for two functional forms of CCL21. These cytokines differ in one amino acid in position 65. Of interest, CCL21-leucine and CCL21-serine are located in separate tissues. CCL21-leu is generally found in non-lymphoid organs (lung, stomach, gut) and CCL21-ser is found in lymphoid tissues (thymus, lymph nodes). In mice, the transition from CCL21-leu to CCL21-ser occurs at the point where lymphatic capillaries in nonlymphoid tissues transition to collecting lymphatic vessels (33). In humans, only the form of CCL21-leu is found and the form CCL21-ser is undetectable (69).
Recently, it was demonstrated that DCs use an amoeboid, integrin-independent mechanism as they home to lymphatic capillaries and migrate to LNs (17, 36). Indeed, pan-integrin deficient DC migrated as well as wild-type controls to the LNs when adoptively transferred (36). For amoeboid mobility, the actin-binding protein Eps8 in DCs plays a critical role to ensure the efficient migration to the local draining LN (17). The expression of the podoplanin receptor Clec2 (encoded in mice by the gene clec1b) was recently reported to be expressed by DCs (1). Clec2-deficient DC display compromised migration to the LNs. Interestingly, the Clec2-podoplanin interaction affected not only the entry of DCs in the lymphatic capillaries but also their crossing of the subcapsular sinus in the LN (1).
3.2 Dendritic cells interaction with the lymphatic collector vessels
Lymphatic collecting vessels are typically surrounded by adipose tissue. This specific adipose tissue contains a large diversity of cell types including macrophages and dendritic cells. Perinodal adipose tissue DCs and macrophages have permanent access to the lymph content that leaks out due to the basal permeability of the lymphatic collecting vessel (33). Furthermore, some of these myeloid cells directly interact with the lymphatic collecting vessel and sample the lymph. However, the reason why this interaction occurs and the physiological relevance of these finding remains unclear. Currently, very little is known about the mechanisms controlling lymphatic vessel permeability. Infection with Yersinia pseudotuberculosis leads to very leaky mesenteric lymphatic collectors (15). As a consequence of the infection, migratory DCs fail to traffic to the local draining LN and this is correlated with subsequent profound defects in the immune response and chronic inflammation. This suggests that lymphatic collecting vessel permeability plays a critical role in the maintenance of cell migration and tissue homeostasis. Interestingly, perinodal adipose tissue IRF4-dependent DCs interact with the lymphatic collecting vessel in a CCR7-dependent manner and maintain basal permeability. The interruption of this interaction leads to collecting vessel leakiness and collagen deposition (27). Morphological changes and increased lymphatic collecting vessel permeability were recently reported in numerous pathologies including hypercholesterolemia and diabetes (39, 57). In the latter, a key molecular player involved is the nitric oxide (NO). NO is produced by endothelial cells via the key enzyme eNOS, by immune cells via iNOS, or by neuronal cells via nNOS. Although the contribution of eNOS in the control of lymphatic collecting vessel permeability was addressed, the relative contributions of iNOS and nNOS remain unknown. How hypercholesterolemia and diabetes affect the distribution and function of perinodal adipose tissue dendritic cells needs further investigation. Additionally, hypercholesteremic ApoE−/− mice, CCR7−/− and IRF4−/− mice all show a clear defect in DC migration (3, 4). The hypothesis that migratory DCs interact with lymphatic collecting vessels during their journey to the LN in order to ensure the maintenance of the integrity and function of the lymphatic vasculature remains an interesting and open question.
4. Concluding remarks
The interaction between immune cells and the lymphatic vasculature is reported in numerous pathological situations including infection, obesity and inflammation. Although significant progress was achieved during the last decade, we only recently shed light on the molecular mechanism involved in this interaction and how both partners are affected when the normal dialogue between myeloid cells and lymphatic endothelial cells is interrupted. Hopefully, an even better understanding of the regulation of this interaction will be obtained soon and this might give us the opportunity to identify new therapeutical targets and develop better strategies to fight and prevent diseases.
Abbreviations
- BATF3
Basic Leucine Zipper ATF-Like Transcription Factor 3
- Csf1r
Colony stimulating factor 1 receptor
- DC
Dendritic cells
- GATA6
GATA Binding Protein 6
- IRF4
Interferon Regulatory Factor 4
- IL
Interleukin
- LN
Lymph node
- LPS
Lipopolysaccharide
- LTA
Lipoteichoic acid
- Lyve1
Lymphatic vessel endothelial hyaluronan receptor 1
- M-CSF
Macrophage colony-stimulating factor
- PAT
Perinodal adipose tissue
- Prox1
Prospero homeobox 1
- TonEBP
Tonicity-responsive enhancer-binding protein
- Vegfr3
Vascular endothelial growth factor receptor 3
Footnotes
Disclosure statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- 1.Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, Jakus Z, Kuligowski M, Fletcher AL, Elpek KG, Bellemare-Pelletier A, Sceats L, Reynoso ED, Gonzalez SF, Graham DB, Chang J, Peters A, Woodruff M, Kim YA, Swat W, Morita T, Kuchroo V, Carroll MC, Kahn ML, Wucherpfennig KW, Turley SJ. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity. 2012;37:276–289. doi: 10.1016/j.immuni.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 3.Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Bajana S, Roach K, Turner S, Paul J, Kovats S. IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation. J Immunol. 2012;189:3368–3377. doi: 10.4049/jimmunol.1102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreau C, Labit E, Guissard C, Rouquette J, Boizeau ML, Gani Koumassi S, Carriere A, Jeanson Y, Berger-Muller S, Dromard C, Plouraboue F, Casteilla L, Lorsignol A. Regionalization of browning revealed by whole subcutaneous adipose tissue imaging. Obesity (Silver Spring) 2016;24:1081–1089. doi: 10.1002/oby.21455. [DOI] [PubMed] [Google Scholar]
- 8.Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol. 1993;67:2209–2220. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgstahler R, Kempkes B, Steube K, Lipp M. Expression of the chemokine receptor BLR2/EBI1 is specifically transactivated by Epstein-Barr virus nuclear antigen 2. Biochem Biophys Res Commun. 1995;215:737–743. doi: 10.1006/bbrc.1995.2525. [DOI] [PubMed] [Google Scholar]
- 11.Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 12.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Fonseca DM, Hand TW, Han SJ, Gerner MY, Glatman Zaretsky A, Byrd AL, Harrison OJ, Ortiz AM, Quinones M, Trinchieri G, Brenchley JM, Brodsky IE, Germain RN, Randolph GJ, Belkaid Y. Microbiota-Dependent Sequelae of Acute Infection Compromise Tissue-Specific Immunity. Cell. 2015;163:354–366. doi: 10.1016/j.cell.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 17.Frittoli E, Matteoli G, Palamidessi A, Mazzini E, Maddaluno L, Disanza A, Yang C, Svitkina T, Rescigno M, Scita G. The signaling adaptor Eps8 is an essential actin capping protein for dendritic cell migration. Immunity. 2011;35:388–399. doi: 10.1016/j.immuni.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautier EL, Ivanov S, Williams JW, Huang SC, Marcelin G, Fairfax K, Wang PL, Francis JS, Leone P, Wilson DB, Artyomov MN, Pearce EJ, Randolph GJ. Gata6 regulates aspartoacylase expression in resident peritoneal macrophages and controls their survival. J Exp Med. 2014;211:1525–1531. doi: 10.1084/jem.20140570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ Immunological Genome C. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon EJ, Rao S, Pollard JW, Nutt SL, Lang RA, Harvey NL. Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development. 2010;137:3899–3910. doi: 10.1242/dev.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, Glass CK. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M, Becher B. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanov S, Scallan JP, Kim KW, Werth K, Johnson MW, Saunders BT, Wang PL, Kuan EL, Straub AC, Ouhachi M, Weinstein EG, Williams JW, Briseno C, Colonna M, Isakson BE, Gautier EL, Forster R, Davis MJ, Zinselmeyer BH, Randolph GJ. CCR7 and IRF4-dependent dendritic cells regulate lymphatic collecting vessel permeability. J Clin Invest. 2016;126:1581–1591. doi: 10.1172/JCI84518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 29.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 30.Kim KE, Koh YJ, Jeon BH, Jang C, Han J, Kataru RP, Schwendener RA, Kim JM, Koh GY. Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol. 2009;175:1733–1745. doi: 10.2353/ajpath.2009.090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KW, Williams JW, Wang YT, Ivanov S, Gilfillan S, Colonna M, Virgin HW, Gautier EL, Randolph GJ. MHC II+ resident peritoneal and pleural macrophages rely on IRF4 for development from circulating monocytes. J Exp Med. 2016;213:1951–1959. doi: 10.1084/jem.20160486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, Mejia C, Frazier WA, Murphy TL, Murphy KM. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC, Randolph GJ. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol. 2015;194:5200–5210. doi: 10.4049/jimmunol.1500221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci U S A. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 37.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KM, Danuser R, Stein JV, Graham D, Nibbs RJ, Graham GJ. The chemokine receptors ACKR2 and CCR2 reciprocally regulate lymphatic vessel density. EMBO J. 2014;33:2564–2580. doi: 10.15252/embj.201488887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim HY, Rutkowski JM, Helft J, Reddy ST, Swartz MA, Randolph GJ, Angeli V. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol. 2009;175:1328–1337. doi: 10.2353/ajpath.2009.080963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 42.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 44.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M Immunological Genome C. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy TL, Grajales-Reyes GE, Wu X, Tussiwand R, Briseno CG, Iwata A, Kretzer NM, Durai V, Murphy KM. Transcriptional Control of Dendritic Cell Development. Annu Rev Immunol. 2016;34:93–119. doi: 10.1146/annurev-immunol-032713-120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ochsenbein AM, Karaman S, Proulx ST, Goldmann R, Chittazhathu J, Dasargyri A, Chong C, Leroux JC, Stanley ER, Detmar M. Regulation of lymphangiogenesis in the diaphragm by macrophages and VEGFR-3 signaling. Angiogenesis. 2016 doi: 10.1007/s10456-016-9523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh YS, Appel LJ, Galis ZS, Hafler DA, He J, Hernandez AL, Joe B, Karumanchi SA, Maric-Bilkan C, Mattson D, Mehta NN, Randolph G, Ryan M, Sandberg K, Titze J, Tolunay E, Toney GM, Harrison DG. National Heart, Lung, and Blood Institute Working Group Report on Salt in Human Health and Sickness: Building on the Current Scientific Evidence. Hypertension. 2016;68:281–288. doi: 10.1161/HYPERTENSIONAHA.116.07415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, Agace WW. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009;206:2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Platt AM, Rutkowski JM, Martel C, Kuan EL, Ivanov S, Swartz MA, Randolph GJ. Normal dendritic cell mobilization to lymph nodes under conditions of severe lymphatic hypoplasia. J Immunol. 2013;190:4608–4620. doi: 10.4049/jimmunol.1202600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ran S, Volk L, Hall K, Flister MJ. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. 2010;17:229–251. doi: 10.1016/j.pathophys.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 55.Rinderknecht M, Detmar M. Tumor lymphangiogenesis and melanoma metastasis. J Cell Physiol. 2008;216:347–354. doi: 10.1002/jcp.21494. [DOI] [PubMed] [Google Scholar]
- 56.Rosas M, Davies LC, Giles PJ, Liao CT, Kharfan B, Stone TC, O’Donnell VB, Fraser DJ, Jones SA, Taylor PR. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–648. doi: 10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scallan JP, Hill MA, Davis MJ. Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovasc Res. 2015;107:89–97. doi: 10.1093/cvr/cvv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schweickart VL, Raport CJ, Godiska R, Byers MG, Eddy RL, Jr, Shows TB, Gray PW. Cloning of human and mouse EBI1, a lymphoid-specific G-protein-coupled receptor encoded on human chromosome 17q12-q21.2. Genomics. 1994;23:643–650. doi: 10.1006/geno.1994.1553. [DOI] [PubMed] [Google Scholar]
- 60.Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, Lippens S, Abels C, Schoonooghe S, Raes G, Devoogdt N, Lambrecht BN, Beschin A, Guilliams M. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:10321. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 62.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139:380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. J Exp Med. 1974;139:1431–1445. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, Pollet E, Ardouin L, Luche H, Sanchez C, Dalod M, Malissen B, Henri S. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Tussiwand R, Lee WL, Murphy TL, Mashayekhi M, Kc W, Albring JC, Satpathy AT, Rotondo JA, Edelson BT, Kretzer NM, Wu X, Weiss LA, Glasmacher E, Li P, Liao W, Behnke M, Lam SS, Aurthur CT, Leonard WJ, Singh H, Stallings CL, Sibley LD, Schreiber RD, Murphy KM. Compensatory dendritic cell development mediated by BATF-IRF interactions. Nature. 2012;490:502–507. doi: 10.1038/nature11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vander Lugt B, Khan AA, Hackney JA, Agrawal S, Lesch J, Zhou M, Lee WP, Park S, Xu M, DeVoss J, Spooner CJ, Chalouni C, Delamarre L, Mellman I, Singh H. Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nat Immunol. 2014;15:161–167. doi: 10.1038/ni.2795. [DOI] [PubMed] [Google Scholar]
- 68.Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–675. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 69.Vassileva G, Soto H, Zlotnik A, Nakano H, Kakiuchi T, Hedrick JA, Lira SA. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J Exp Med. 1999;190:1183–1188. doi: 10.1084/jem.190.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weinkopff T, Konradt C, Christian DA, Discher DE, Hunter CA, Scott P. Leishmania major Infection-Induced VEGF-A/VEGFR-2 Signaling Promotes Lymphangiogenesis That Controls Disease. J Immunol. 2016;197:1823–1831. doi: 10.4049/jimmunol.1600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 73.Wiig H, Schroder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Muller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witmer-Pack MD, Hughes DA, Schuler G, Lawson L, McWilliam A, Inaba K, Steinman RM, Gordon S. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J Cell Sci. 1993;104(Pt 4):1021–1029. doi: 10.1242/jcs.104.4.1021. [DOI] [PubMed] [Google Scholar]
- 75.Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010;207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang H, Kim C, Kim MJ, Schwendener RA, Alitalo K, Heston W, Kim I, Kim WJ, Koh GY. Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol Cancer. 2011;10:36. doi: 10.1186/1476-4598-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu XM, Lo CY, Lam AK, Lang BH, Leung P, Luk JM. The potential clinical relevance of serum vascular endothelial growth factor (VEGF) and VEGF-C in recurrent papillary thyroid carcinoma. Surgery. 2008;144:934–940. doi: 10.1016/j.surg.2008.07.027. discussion 940–931. [DOI] [PubMed] [Google Scholar]