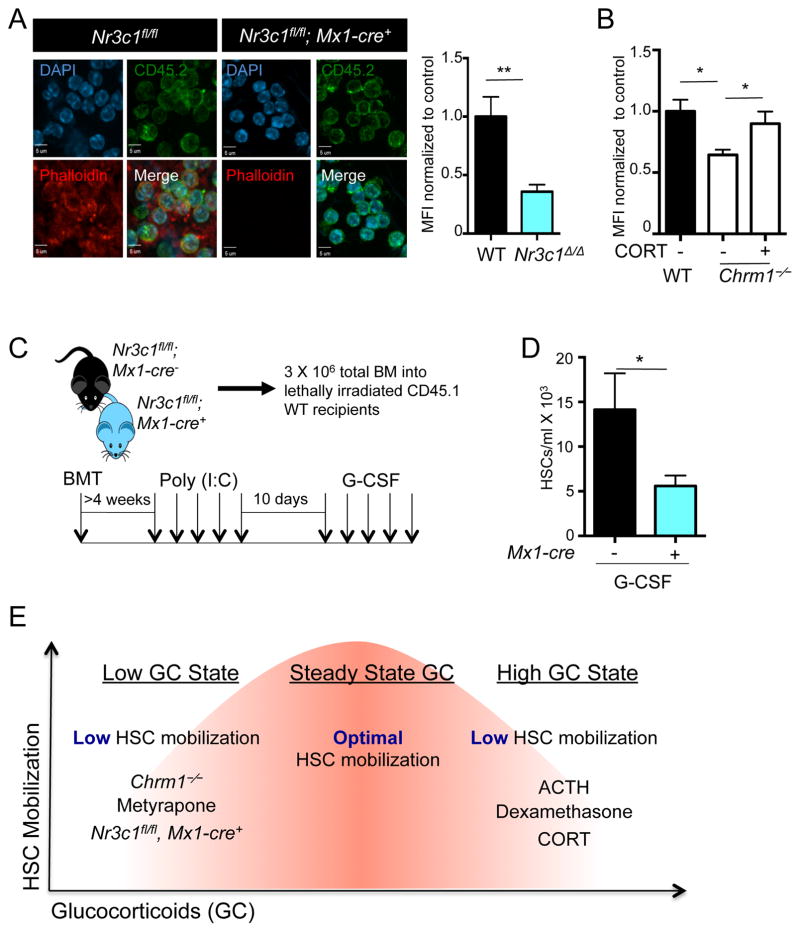
Figure 6. Nr3c1 expression on hematopoietic cells is required for enforced G-CSF-induced migration.
(A) Representative images of leukocytes stained for phalloidin (polymerized actin) and CD45.2 (donor cells) obtained from radiation chimeras generated by transplantation of control (Nr3c1fl/fl) or Nr3c1-deficient (Nr3c1fl/fl; Mx1-cre+) BM into lethally irradiated C57BL/6 mice and quantification of phalloidin staining normalized to control MFI (n=3).
(B) Quantification of phalloidin staining in Chrm1−/− cells and Chrm1−/− cells taken from animals treated with corticosterone normalized to control MFI (n-3).
(C) Schematic of experimental design for G-CSF-induced mobilization.
(D) G-CSF mobilized HSCs in control (Nr3c1fl/fl) and Nr3c1-deficient (Nr3c1fl/fl; Mx1-cre+) mice (n = 6–11).
(E) Model whereby finely tuned GC levels regulate HSC migration. States associated with low GCs (Chrm1−/− mice, Metyrapone administration, Nr3c1-deficient HSCs) or high GCs (administration of ACTH, Dexamethasone or corticosterone) lead to reduced mobilization efficiency.
* p < 0.05 ** p < 0.01 determined by Student’s t test. Data are represented as mean ± SEM.