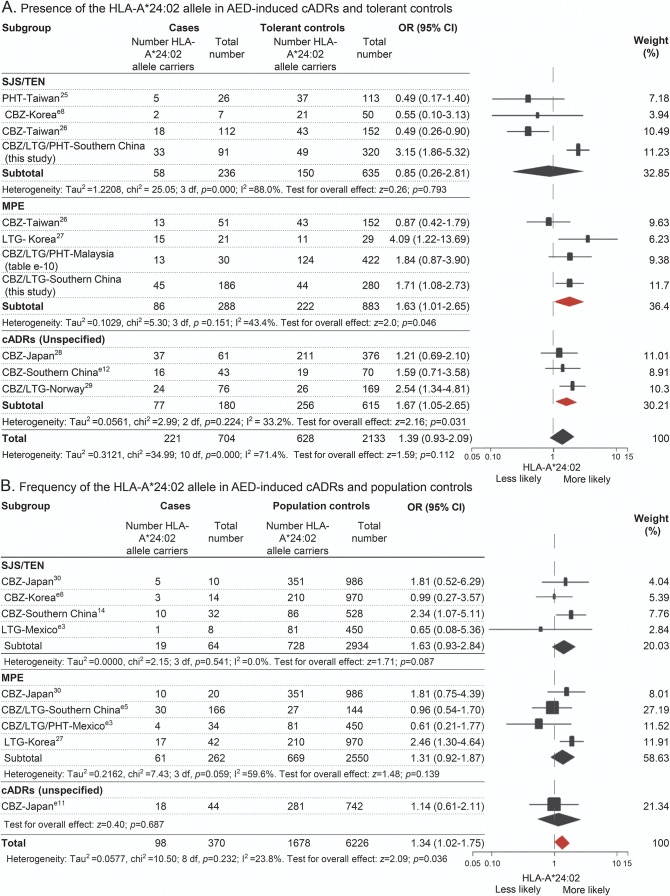
Figure 2. Distribution of HLA-A*24:02 allele across the phenotypes of cADRs induced by aromatic AEDs.
Data were from 14 studies, including 7 that used drug-tolerant controls, 5 that used population controls, and 2 that used both types of controls, including populations from Japan, Korea, Malaysia, Mexico, Norway, and China (e-supplement). Data from tolerant controls (A) and population controls (B) were analyzed separately. The phenotypes of cADRs included SJS/TEN, MPE, and unspecified cADRs, according to the descriptions in the original reports. I2 and τ2 and represent measures of heterogeneity. Study weighting (indicated by different sizes of squares) refers to the proportion of participants who were recruited from each study. Horizontal lines represent 95% CIs; diamonds indicate pooled ORs. AED = antiepileptic drug; cADRs = cutaneous adverse drug reactions; CBZ = carbamazepine; CI = confidence interval; HLA = human leukocyte antigen; LTG = lamotrigine; MPE = maculopapular exanthema; OR = odds ratio; PHT = phenytoin; SJS/TEN = Stevens-Johnson syndrome/toxic epidermal necrolysis.