Figure 1.
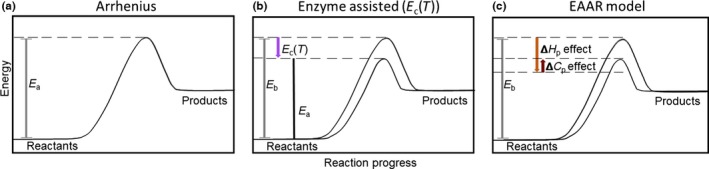
(a) Reactions proceed as the reactants gain enough energy to clear the hurdle of the activation energy (E a) to form products. (b) Organisms contribute some energy to reactions occurring within their bodies with enzymes. The contributed energy lowers the kinetic hurdle that reactants must clear, such that the net activation energy is E b − E c(T), the latter of which is temperature dependent via effects of temperature on protein stability. (c) Metabolic reactions have to clear their particular activation energy (E b, gray bar), but enzymes provide a temperature‐dependent contribution to the starting energetic state of the reactants through a temperature‐dependent increase in stability (ΔH, orange arrow). Below the melting temperature, however, temperature lowers the energetic state through its effect on heat capacity (ΔCp, red arrow)
