Figure 4.
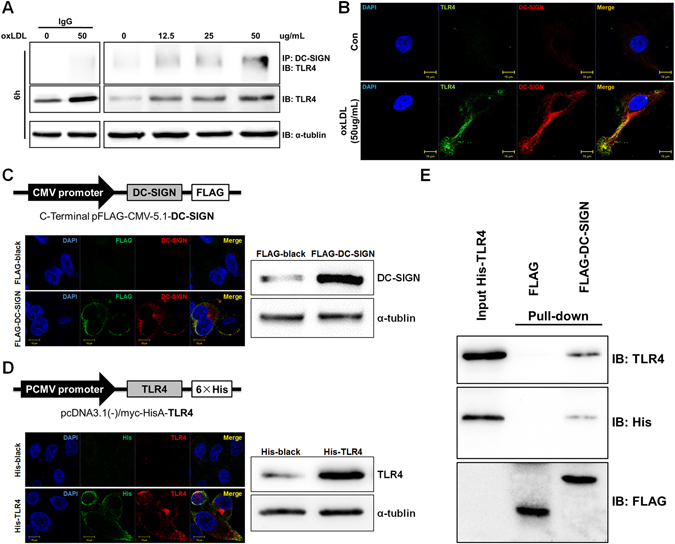
DC-SIGN interacted with and bound TLR4. (A) Macrophages were incubated with oxLDL (0, 12.5, 25 and 50 μg/ml) for 6 hours. Cell lysates were immunoprecipitated with a DC-SIGN antibody and probed with an antibody against TLR4. As loading controls, whole cell lysates were probed with antibodies against total TLR4 and α-tubulin. The IgG control had been detected in another Western-blot assay, where the protein level and exposure time were same Western-blot results on the right. (B) Macrophages were stimulated with or without 50 μg/ml oxLDL and stained with DC-SIGN (red) and TLR4 (green). Images were acquired by confocal microscopy (1,200x). Yellow indicates co-localization of the two proteins. (C and D) The maps of pFLAG-CMV-5.1-DC-SIGN and pcDNA3.1 (−)/myc-HisA-TLR4 are shown. pFLAG-CMV-5.1-DC-SIGN or pcDNA3.1 (−)/myc-HisA-TLR4 were transfected into HEK293 cells. The localization patterns of FLAG-DC-SIGN (FLAG: green, DC-SIGN: red) and His-TLR4 (His: green, TLR4: red) were detected by immunofluorescence stains, and the overexpression efficiency of FLAG-DC-SIGN and His-TLR4 were measured by western blot analysis. (E) In vitro pull-down of FLAG-DC-SIGN and His-TLR4 fusion proteins. The total cell lysate (10 μg) of FLAG and FLAG-DC-SIGN were absorbed onto anti-FLAG M2 beads and incubated with the whole cell lysate (10 μg) His-TLR4. Elutes were analyzed by SDS-PAGE followed by immunoblotting with anti-TLR4, anti-His and anti-FLAG. IB: Immunoblotting, IP: Immunoprecipitation.
