Fig. 6.
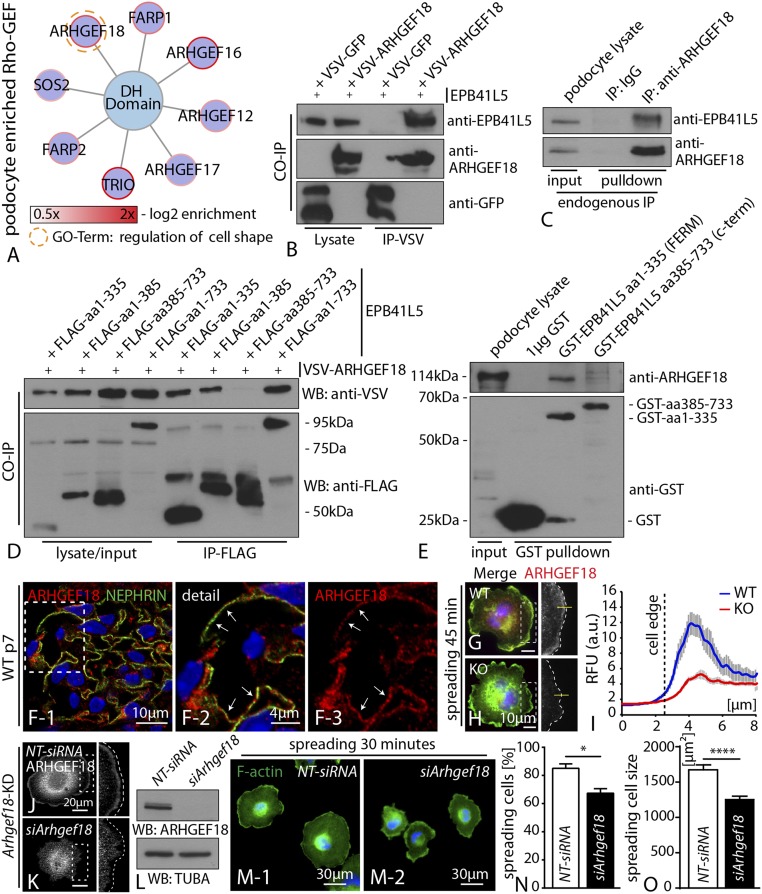
EPB41L5 regulates actomyosin contractility via ARHGEF18. (A) Subanalysis of the in vivo iTRAQ proteomics dataset for DH domain containing small GTPases. ARHGEF18 showed a high enrichment score, as well as involvement in cell shape regulation. (B) Coimmunoprecipitation between epitope-tagged EPB41L5 and ARHGEF18. (C) Endogenous EPB41L5 was precipitated via pull-down with an antibody directed against ARHGEF18, IgG was included as a control. (D) A series of different EPB41L5 truncations (all epitope tagged) were used to map the association with ARHEF18; here only the FERM domain containing truncations precipitated ARHGEF18. (E) GST-tagged recombinant protein versions of either FERM domain or C-terminal truncations of EPB41L5 were used in endogenous pull-down experiments. Only the FERM domain containing truncation precipitated ARHGEF18. (F) Immunofluorescence staining for ARHGEF18 on murine adult glomeruli revealed colocalization with the podocyte marker NEPHRIN (boxed regions indicate zoomed-in detail). (G–I) Immunofluorescence staining for ARHGEF18 on cells, while spreading revealed localization toward the leading edge. Quantification of immunofluorescence intensities across the cell border indicated decreased levels ARHGEF18 in KO cells (at least 20 cells per genotype were analyzed over two independent experiments). (J–L) siRNA-mediated knockdown of ARHGEF18 in immortalized human podocytes, as confirmed via immunofluorescence and Western blot. (M–O) Knockdown of ARHGEF18 resulted in significant impairment of early spreading on collagen IV surfaces (at least 100 cells per condition were analyzed and averaged over three independent experiments; Dataset S3).