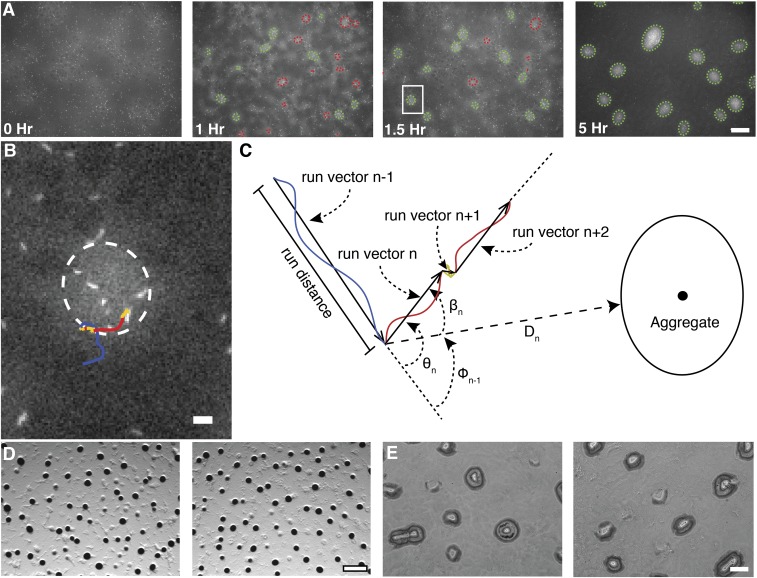
Fig. S1.
Overview of individual cell and aggregate tracking. (A) Representative images from time-lapse microcinematography of developing M. xanthus cells highly expressing tdTomato mixed 1:2,500 with cells weakly expressing eYFP. Cell density is proportional to eYFP fluorescence intensity whereas, in the same image, individual tdTomato cells are bright enough to detect and track . Detected aggregate boundaries are indicated with dashed green ellipses for stable aggregates and red ellipses for unstable aggregates. (Scale bar, 100 μm.) (B) Increased magnification of the image area inside the white box in A. Line follows a single cell trajectory from the prior 40 min to the shown frame. Line color indicates detected cell state. Blue is persistent forward, red is persistent backward, and yellow is nonpersistent movement. (Scale bar, 10 μm.) (C) Cell trajectories were segmented into continuous states, and the vector pointing from one state to the next is defined as a run vector. Colors are as in B. Run vector distance, speed, duration, distance to nearest aggregate boundary (), angle between two consecutive run vectors and the angle between the nearest aggregate centroid and the ending of the previous and beginning of the run vectors are representative of the variables calculated. All angles are in the interval [), where (D) Mixtures of LS3629 and LS3908 on plates containing IPTG and vanillate (Left) or DK1622 cells without IPTG or vanillate (Right) produced similar aggregate profiles. Images were taken 48 h poststarvation at 25× magnification. (Scale bar, 500 μm.) (E) Aggregation profiles were similar after 5 h of fluorescent imaging (Left) and without any fluorescent imaging (Right). The phase images were taken at the same time point and magnification as the 5-h panel in A. (Scale bar, 100 μm.)