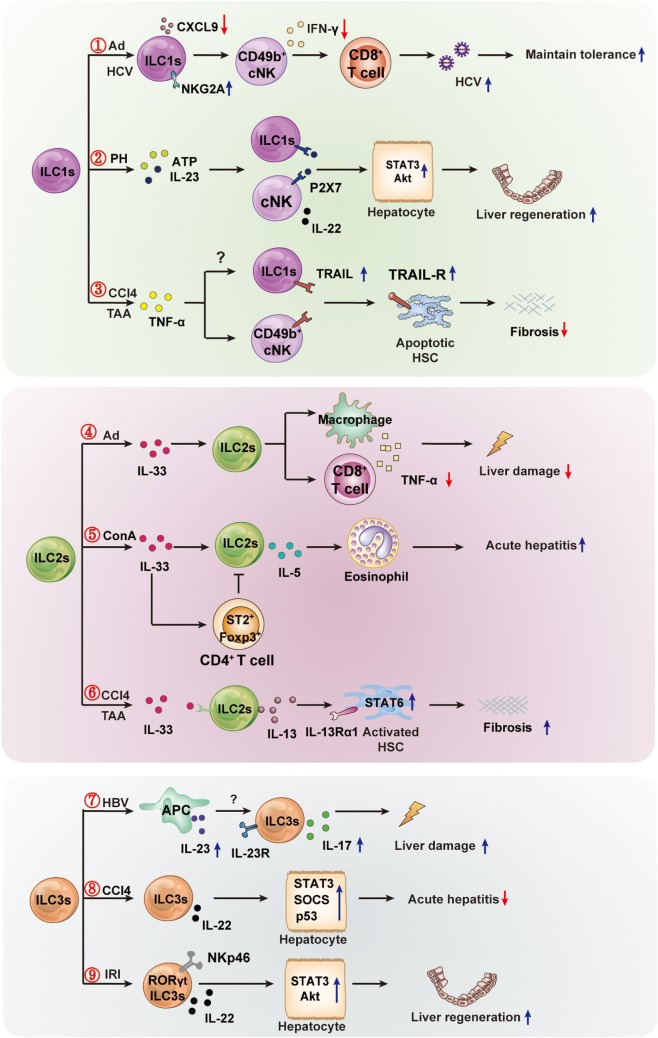
Figure 2.
The protective or pathogenic roles of innate lymphoid cells (ILCs) in liver diseases. The hepatic ILC subsets are involved in the immune regulation of liver diseases (viral hepatitis, mechanical liver injury, and fibrosis). ① In hepatic adenovirus (Ad) or HCV infection, hepatic ILC1s play an important role in maintaining liver tolerance. Hepatic viral infection increases NKG2A expression on ILC1s, and NKG2A signaling in ILC1s inhibits CXCL9 expression, which is required for the accumulation of IFN-γ+CD49b+ NK cells (cNK cells). This, in turn, results in the loss of IFN-γ production, which is crucial for the enhanced priming of CD8+ T cells. ② Hepatic ILC1s and cNKs contribute to liver regeneration. cNK cells and ILC1s produce a high level of IL-22 in response to elevated adenosine triphosphate (ATP) and IL-23 in an ATP receptor P2X1 (P2-type nucleotide receptors)-dependent manner; IL-22 promotes hepatocyte growth via activation of the STAT3 pathway. ③ Hepatic cNK or ILC1s limit liver fibrosis. The inflammatory cytokine TNFα increases expression of TNF-related apoptosis-inducing ligand (TRAIL) on cNK cells, and then enhances cNK cell-mediated hepatic stellate cell (HSC) killing. The high expression of TRAIL may lead to hepatic ILC1s having similar effects to the killing of activated HSC, thus limiting liver fibrosis. ④ In Ad-induced liver inflammation, ILC2s exhibit a hepatoprotective role. The expression of IL-33 and its receptor ST2 in the liver are increased, and the ILC2s expand in response to IL-33 and limit liver injury by suppressing TNFα production in hepatic T cells and macrophages. ⑤ In Con A-induced hepatitis, hepatic ILC2s are activated and expanded in response to IL-33, further amplifying inflammatory immune responses via IL-5-mediated recruitment of eosinophils. The inflammatory activity of ILC2s might be regulated by IL-33-expanded CD4+Foxp3+ regulatory T cells (Treg). ⑥ In CCl4-or thioacetamide (TAA)-induced chronic hepatocellular stress, IL-33 activates ILC2s, producing IL-13, leading to HSC activation through IL-13Rα1- and STAT6-dependent signaling. ⑦ Hepatitis B virus infection induces IL-23 production by antigen-presenting cells, and the increased IL-23 contribute to liver damage via IL-17 production, possibly by activating the IL-23 receptor-expressing ILC3s. ⑧ In CCl4-induced acute hepatitis, the IL-22-producing ILC3s inhibit liver injury via IL-22 production. ⑨ In the pathogenesis of hepatic ischemia reperfusion injury (IRI), RORγt-expressing NKp46+ cells are capable of ameliorating hepatic IRI in an IL-22-dependent manner.