Abstract
Chromatin modification enzymes are important regulators of gene expression and some are evolutionarily conserved from yeast to human. Saccharomyces cerevisiae is a major model organism for genome-wide studies that aim at the identification of target genes under the control of conserved epigenetic regulators. Ume6 interacts with the upstream repressor site 1 (URS1) and represses transcription by recruiting both the conserved histone deacetylase Rpd3 (through the co-repressor Sin3) and the chromatin-remodeling factor Isw2. Cells lacking Ume6 are defective in growth, stress response, and meiotic development. RNA profiling studies and in vivo protein-DNA binding assays identified mRNAs or transcript isoforms that are directly repressed by Ume6 in mitosis. However, a comprehensive understanding of the transcriptional alterations, which underlie the complex ume6Δ mutant phenotype during fermentation, respiration, or sporulation, is lacking. We report the protein-coding transcriptome of a diploid MATa/α wild-type and ume6/ume6 mutant strains cultured in rich media with glucose or acetate as a carbon source, or sporulation-inducing medium. We distinguished direct from indirect effects on mRNA levels by combining GeneChip data with URS1 motif predictions and published high-throughput in vivo Ume6-DNA binding data. To gain insight into the molecular interactions between successive waves of Ume6-dependent meiotic genes, we integrated expression data with information on protein networks. Our work identifies novel Ume6 repressed genes during growth and development and reveals a strong effect of the carbon source on the derepression pattern of transcripts in growing and developmentally arrested ume6/ume6 mutant cells. Since yeast is a useful model organism for chromatin-mediated effects on gene expression, our results provide a rich source for further genetic and molecular biological work on the regulation of cell growth and cell differentiation in eukaryotes.
Keywords: Ume6, Rpd3, Sin3, Isw2, Transcriptome, Interactome
Introduction
Chromatin modification enzymes are part of multi-subunit regulatory complexes, which control gene expression in eukaryotic cells. Identifying their target promoters at the genome-wide level helps understand their roles and contributes to future work that aims at a system-level understanding of processes that govern cell growth and development. The budding yeast Saccharomyces cerevisiae is a very useful model organism for genetic and genomic studies that aim at a better understanding of transcriptional regulatory mechanisms, which involve conserved proteins.
UME6 (Unscheduled Meiotic Expression 6) was initially identified in a screen for haploid mutants that express meiosis-specific genes during vegetative growth in S. cerevisiae (Strich et al. 1989). The gene is conserved among fungi where it plays important roles in establishing cellular identity in K. lactis and controlling filamentous growth in the human pathogen C. albicans (Carlisle et al. 2009; Zeidler et al. 2009; Carlisle and Kadosh 2010; O’Connor et al. 2010; Banerjee et al. 2013; Childers et al. 2014). Mutant budding yeast cells lacking UME6 show a pleiotropic phenotype including defective mitotic growth, abnormal vacuolar fragmentation, broadly impaired stress response, and failure to enter meiotic M-phase (Strich et al. 1994; Hillenmeyer et al. 2008; Yoshikawa et al. 2011; Michaillat and Mayer 2013). In addition to genes required for metabolic functions and meiosis, Ume6 also represses ATG8, which is important for autophagy, a conserved process that recycles cellular components (Tsukada and Ohsumi 1993; Kratzer and Schuller 1997; Messenguy et al. 2000; Bartholomew et al. 2012). Recently, we reported a new role for Ume6 in preventing the expression of early meiosis-specific transcript isoforms with an extended 5′-untranslated region (UTR) during rapid mitotic growth (Lardenois et al. 2015).
In budding yeast, Ume6 is regulated at the post-transcriptional level at three stages. First, the Spt-Ada-Gcn5-acetyltransferase (SAGA) complex acetylates Ume6 when cells switch from fermenting glucose to respiring acetate conditions, and thereby diminishes its DNA binding activity. A second acetylation event stimulates protein degradation by the anaphase promoting complex/cyclosome (APC/C) ubiquitin ligase and Inducer of Meiosis 1 (Ime1) during the onset of meiotic development. Ume6 levels remain below the limits of detection during the meiotic divisions before it accumulates again at late stages of sporulation to ultimately exert a function during spore germination (Mallory et al. 2007, 2012; Strich et al. 2011; Law et al. 2014).
Ume6 is a C6 zinc cluster protein, which directly binds the upstream repressor site 1 (URS1) motif (5′-TAGC-CGCCGA-3′) present in single or multiple copies within target promoters (Park et al. 1992; Anderson et al. 1995), for review, see (Mitchell 1994; Kassir et al. 2003). Initially, URS1 sites were identified using sequence homology searches within the 5′-upstream regions of potential target genes (Williams et al. 2002). When several genomes of related yeasts were sequenced, discovering regulatory DNA elements such as URS1 was facilitated because transcription factor binding sites located within intergenic regions were conserved (Cliften et al. 2003; Kellis et al. 2003). Moreover, genome-wide protein-DNA binding assays identified direct targets for most regulatory proteins, including Ume6, in yeast (Kurdistani et al. 2002; Harbison et al. 2004). A current method to analyze promoter regions employs position weight matrices (PWMs), which are established by aligning experimentally verified binding sites of transcription factors (TFs), and log-transforming the number of observations of each nucleotide within their target motifs (Orenstein et al. 2012). PWMs are available for nearly all yeast TFs via the TRANSFAC database (Wingender 2008; Spivak and Stormo 2012).
Ume6-dependent transcriptional repression is mediated by recruitment of the conserved histone deacetylase Rpd3 through the co-repressor Sin3 and the chromatin-remodeling factor Isw2 (Anderson et al. 1995; Kadosh and Struhl 1997; Rundlett et al. 1998; Goldmark et al. 2000). In addition, biochemical studies and high-throughput assays have shown that Ume6 interacts with many proteins both physically and genetically (for more details, see www.ebi.ac.uk/intact/) (Baryshnikova et al. 2010; Ngounou Wetie et al. 2014; Orchard et al. 2014). The yeast interactome includes physical interactions established via yeast two-hybrid assays (direct binding) and co-immunoprecipitation (direct and indirect binding in a complex) and genetic interactions determined by screening double mutants for growth phenotypes. Integrating expression profiling data with protein network information provides insight into the dynamic nature of protein interactions during normal growth or cell differentiation pathways (Prinz et al. 2004; de Lichtenberg et al. 2005).
In this study, we compared the protein-coding transcriptome in triplicate samples from a wild-type diploid strain to a ume6 mutant strain cultured in rich fermentation or respiration conditions (YPD or YPA, respectively), or in sporulation medium, which lacks both glucose and nitrogen (SPII). In addition to asynchronously growing diploid cells, we used published data from synchronized MATa cells progressing through the mitotic cell cycle to determine which Ume6-dependent mRNAs induced during sporulation are undetectable in both haploid and diploid cells cultured in YPD (Orlando et al. 2008). We further differentiated between direct and indirect effects by combining GeneChip data with URS1 motif predictions and published high-throughput in vivo Ume6-DNA binding data (Harbison et al. 2004). For a better understanding of the physical and genetic interactions between successive waves of meiotic genes, we integrated expression data with information on protein networks. Using this integrative approach, we identify new Ume6-dependent genes, and demonstrate that the nutritional status of the cells affects the derepression of most Ume6 target genes. These expression data, which are available for further analyses at the EBI’s ArrayExpress (Rustici et al. 2013), and for viewing in the GermOnline database (www.germonline.org) (Lardenois et al. 2010), represent a rich source for further investigation of epigenetic control mechanisms governing yeast cell growth and differentiation.
Materials and methods
Yeast strains
We employed a diploid SK1 MATa/α wild-type strain and a diploid SK1 MATa/α ume6/ume6 mutant strain as published (Williams et al. 2002).
Media and culture conditions
For RNA profiling experiments, three independent samples of wild-type versus mutant strains were cultured in rich media with glucose (YPD) or acetate (YPA) or sporulation medium (SPII at 4, 8, and 10 h) under standard conditions as published (Primig et al. 2000).
Total RNA isolation and cRNA target synthesis
5 μg of total RNA was subjected to double-stranded cDNA synthesis using the One-Cycle cDNA synthesis kit (Affymetrix, Santa Clara, USA). The cDNA was purified with the Sample Cleanup Module (Affymetrix) and used to synthesize cRNA in the presence of a biotin-conjugated ribonucleotide analog with the IVT labeling kit (Affymetrix). Approximately 45 μg of labeled cRNA from each reaction was purified and the average size of the cRNA molecules was assessed with a BioAnalyzer and RNA Nano 6000 Chips (Agilent, Santa Clara, USA). cRNA targets were incubated at 94 °C for 35 min in fragmentation buffer and the resulting fragments of 50–150 nucleotides were again monitored using the BioAnalyzer. Synthesis reactions were carried out using a T1 Thermocycler (Biometra, Göttingen, Germany). 80 μl hybridization cocktail containing fragmented biotin-labeled target cRNA at a final concentration of 0.05 μg/μl was loaded into Yeast Genome 2.0 Gene-Chips (Affymetrix) and incubated at 45 °C in a hybridization oven 640 (Affymetrix) for 16 h at 60 rpm.
GeneChip hybridization and raw data production
The arrays were washed and stained on a Fluidics Station 450 (Affymetrix) using the Hybridization Wash and Stain Kit (Affymetrix). To increase the signal strength, we employed the antibody amplification protocol (FS450_0003). The GeneChips were processed with a GeneChip Scanner 3000 (Affymetrix) using the default settings. Raw data CEL files were generated using GeneChip Operating Software GCOS 1.4 (Affymetrix).
Minimum information about a microarray experiment (MIAME) compliance
A complete set of raw data files is available at the European Bioinformatics Institute’s ArrayExpress certified repository; the accession number is E-TABM-192 (Rustici et al. 2013). In addition, graphical displays of the normalized GeneChip signals in the context of yeast genome annotation data are available at GermOnline (www.germonline.org) (Lardenois et al. 2010).
GeneChip data processing
Yeast Genome 2.0 GeneChip data were processed and normalized using the AMEN software tool as published (Chalmel et al. 2007).
Genome-wide in vivo Ume6 binding data
We integrated the output of a Ume6 chromatin immunoprecipitation—chip (ChIP-Chip) assay done with haploid W303 yeast strain grown in rich medium (YPD) (Harbison et al. 2004).
URS1 motif predictions
The Ume6 target site URS1 was predicted at a genome-wide level in reference (Lardenois et al. 2015).
Gene filtration and cluster analysis
First, we identified 1571 probesets (corresponding to 1560 genes) defined as differentially expressed within a sample set from the diploid wild-type strain (UME6), the ume6 deletion mutant (ume6) and the combined set using a standard deviation ≥1.0. Second, we filtered 3139 probesets (3095 genes) showing a fold-change ≥2 in paired wild-type versus mutant samples. The intersection of both lists yielded 1404 probesets (1393 genes). To determine the statistical significance of their signal variations, we used permutation tests [p value (FDR) ≤ 0.01], which yielded 1399 probesets (1390 genes). Finally, these genes were grouped according to their expression patterns into 12 clusters using the partitioning around medoids (PAM) algorithm as published (Online Resource Figure S5) (Chalmel et al. 2007).
Gene ontology term enrichment
For each cluster, a Gene Ontology (2015) term enrichment was performed via DAVID (da Huang et al. 2009). The biological process (BP) sub-ontology was used to identify the functional pathways over-represented in each group, using a hyper-geometric law with Benjamini correction for multiple testing, and the whole yeast genome as reference dataset. The BP terms with a p value <0.01 % were then clustered with a medium stringency and each cluster received as name the BP annotation representing more than 10 % of the cluster with the strongest p value.
Protein network analysis
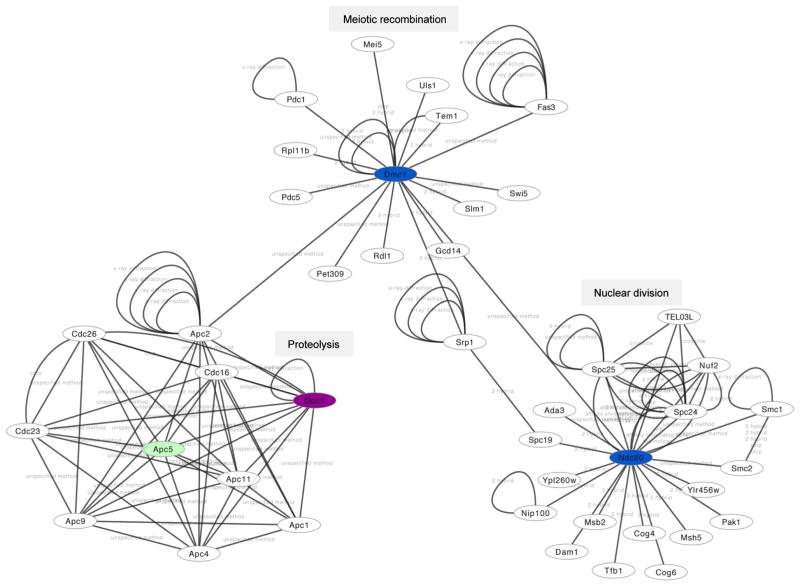
Protein network analysis was performed using CytoScape version 3.2.0. (Saito et al. 2012). The network was extracted from BIND (Isserlin et al. 2011) using the dedicated Cytoscape interface. The analysis was centered at genes differentially expressed and not expressed during wild-type mitosis (such genes were identified in clusters G9, G8, G1, and G10), as well as their direct interactors. We identified 14 connected components (CCs): two of them have more than 20 members; among them more than 10 are differentially expressed and not expressed during mitosis. One CC is presented in Fig. 6 to illustrate the approach.
Fig. 6.
Protein network interactions. A protein network view created with Cytoscape 3.2.0. is shown. Ume6 target proteins that are repressed in mitosis and that fall into cluster 8 and 9 are shown in magenta and blue, respectively. Apc5, shown in green, is among indirect targets. The network annotation data provided by BIND (see “Materials and methods”) were manually edited for clarity. We note that some complexes refer to publications that report the interaction but do not provide information about the experimental approach used to detect it. Such cases are termed unspecified (interaction detection) method
Chromatin immunoprecipitation
In vivo Ume6-DNA binding to the SIP4 promoter was monitored in cells cultured in rich media (YPD, YPA) or sporulation medium (SPII) at 4, 8, and 10 h using the strain background described in reference (Lardenois et al. 2015). 50 ml of a mid-log YPA culture was fixed with 1 % formaldehyde for 15 min at room temperature. Ume6/DNA complexes were quenched with 140 mM glycine (Sigma, St Louis USA) for 5 min. Anti-Ume6 immune complexes were immunoprecipitated, washed, and eluted before crosslinks were reverted. DNA was precipitated and treated with proteinase K. DNA fragments were amplified by Q-PCR as published (Mallory et al. 2012). Relative ChIP signals were calculated using the formula 2^-IP (CT target–CT control)/input (CT target–CT control) with NUP85 enrichment used as a negative control. Binding to SPO13 was used as a positive control as published (Law et al. 2014). The oligonucleotide primers used to amplify the SIP4 promoter were 5′-GCCGTTCGACCGGTGTT-3′ (forward) and 5′-TTTGCCGCCGAGTTCTG-3′ (reverse). For SPO13, we used 5′-GCTAGTTAGTACCTTTGCACGGAAA-3′ (forward) and 5′-TCTTATTGCGCTAATTGTCTGTTAGAC-3′ (reverse).
RT-PCR assays
Yeast Genome 2.0 GeneChip data were validated by RT-PCR assays as published (Lardenois et al. 2015). Total RNA was isolated using the hot phenol method as described (Primig et al. 2000). Briefly, cell pellets were treated with hot phenol (65 °C) and phenol/chloroform (1:1). Total RNA was precipitated overnight with two volumes of 100 % ethanol and 0.1 volume of 3 M NaOAc (pH 5) at −80 °C. The RNA was digested with 2 units of DNaseI for 30 min at 37 °C, and then 2 μg of DNaseI-treated RNA was reverse transcribed into cDNA using reverse transcriptase and random primers supplied in the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). 1 μl of cDNA was amplified at 28 cycles (denaturation at 94 °C for 1 min, annealing at 60 °C for 1 min, and elongation at 72 °C for 1 min) using Taq DNA Polymerase (Qiagen). The PCR product was run on a 2 % agarose gel in 1 × TAE buffer containing GelRed DNA dye (Biotium) and photographed using the Gel Doc XR+ imaging system (BIO-RAD). The sequences of oligonucleotide primers used for cDNA amplification were 5′-ATTGGCGACCTGGAAATGGA-3′ (CSM4, forward); 5′-TGAACACACCTCATCGCTCAA-3′ (CSM4, reverse); 5′-GGACTTGACCTTTGGGGGAG-3′ (FKS3, forward); 5′-AGACGTTCCAAAACCTCGCT-3′ (FKS3, reverse); 5′-GGACCCTCCTCAATCAAGCC-3′ (HFM1, forward); and 5′-ACTTGTTCACCCGCTTCCAT-3′ (HFM1, reverse).
Results
Experimental design and quality control
We RNA-profiled key stages of the diploid yeast life cycle in the presence or absence of Ume6-dependent epigenetic chromatin modification using robust Yeast Genome 2.0 GeneChips that cover nearly all protein-coding genes. We note that the mRNA data generated by these microarrays are comparable to the most recent expression profiling methods based on RNA-Sequencing (Nookaew et al. 2012). We analyzed triplicate biological samples from asynchronous fermenting (YPD) or respiring (YPA) diploid SK1 MATa/α cells harvested during late-log phase and semi-synchronous sporulating cells 4, 8, and 10 h after meiotic induction. At these time points cells progress through meiosis I prophase (4 h), meiosis I (8 h), or meiosis II (10 h), respectively (supplementary Online Resource Figure S1). The output from these experiments was combined with published Yeast Genome 2.0 expression data from haploid BF264-15Dau MATa cells undergoing synchronous mitotic cell cycles in rich medium (YPD) (Orlando et al. 2008). We identified transcripts expressed during meiotic development that are undetectable in fermenting and respiring diploid MATa/α cells and during successive phases of the mitotic cell cycle in fermenting haploid MATa cells. Total RNA samples from the wild-type strain and the ume6/ume6 mutant were of high quality (Online Resource Figure S2 and Online Resource Figure S3), and the microarray signal intensity distributions fell into broadly similar ranges for each sample, indicating that the GeneChip hybridization reactions yielded data that are comparable once normalized (Online Resource Figure S4A, B).
Genome-wide expression signal distributions across samples reflect the diploid ume6/ume6 mutant phenotype
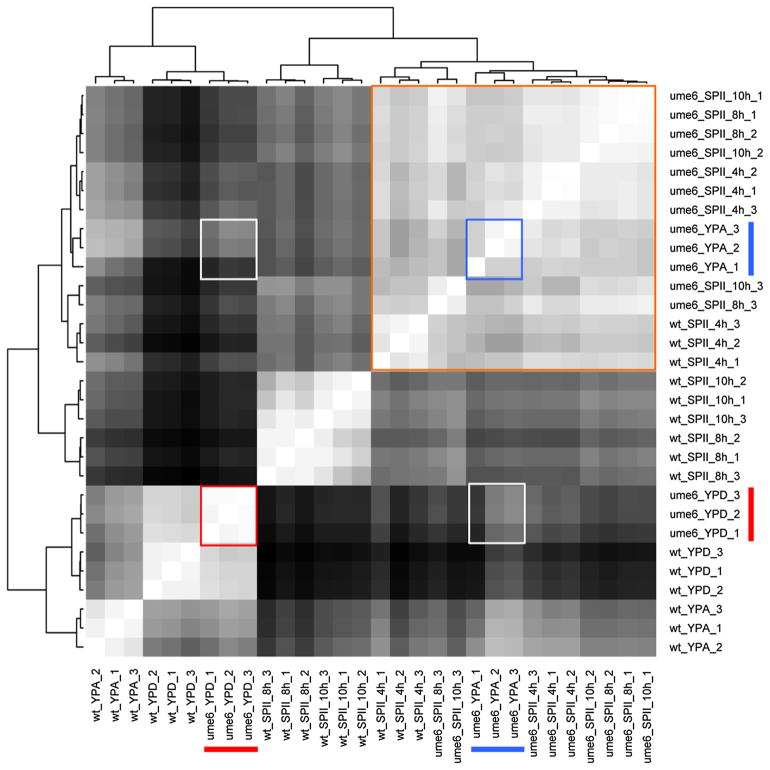
To determine the degree of reproducibility between biological replicates, we compared their global signal patterns to each other using a correlation distance matrix. As expected, wild-type replicates cultured in YPD, YPA, or SPII were consistently grouped together and distinct signal patterns emerged as cells exit growth and enter meiotic differentiation (Fig. 1). Conversely, samples from ume6/ume6 mutant cells cultured in pre-sporulation medium (YPA) and sporulation medium (SPII) are broadly similar (note that replicates at SPII 8 h and 10 h are interspersed) and grouped with the wild-type in SPII 4 h. This sample broadly corresponds to the end of pre-meiotic DNA replication, which is the phase where mutant cells accumulate (Strich et al. 1994). The plot also highlights that ume6/ume6 mutants show distinct global profiles in the presence of glucose and acetate, which indicates that the pattern of gene derepression during vegetative growth is widely influenced by the carbon source (Fig. 1).
Fig. 1.
Expression signal comparison across samples. A distance matrix comparing the samples from wild-type (wt) cells and a ume6/ume6 mutant (ume6) in growth media (YPD, YPA) and sporulation medium (SPII 4, 6 and 8 h) is shown. Red and blue bars indicate triplicate samples from ume6/ume6 cells in YPD and YPA, respectively. Red and blue squares indicate samples comparisons among replicates in YPD or YPA. White squares indicate sample comparisons in YPD against YPA. An orange square outlines similar samples across media and strains. On the color scale white represents identical samples and black identifies the most dissimilar samples.
Global RNA profiling of growth and development in the absence of Ume6 reveals known and new patterns of de-regulation
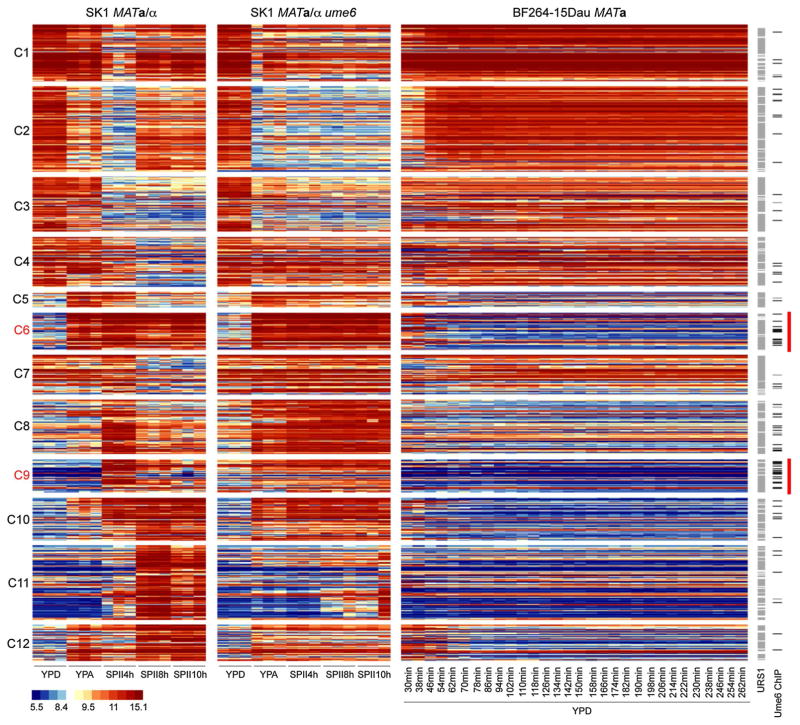
Our approach was designed to cover changes in RNA levels during growth and development related to the deletion of UME6 as broadly as possible. As a consequence, we identified all core Ume6 target genes determined in an earlier RNA profiling study (Williams et al. 2002), apart from the dubious gene YBR116C not represented on the Yeast Genome 2.0 GeneChip, and YIR016W and YMR101C that are not differentially expressed in the current study. We filtered 1390 transcripts showing differential expression signals across the sample sets (Online Resource Figure S5; see “Materials and methods” for more details on the procedure) and grouped them into twelve clusters according to their peak expression in YPD, YPA, or SPII media (Fig. 2, Online Resource File S1). Clusters 1–2 contain genes actively transcribed during vegetative growth and meiotic M-phase in the wild-type but not in the ume6/ume6 mutant that arrests before entering M-phase. Clusters 3–7 comprise genes that are progressively down-regulated during sporulation in the wild-type but that show altered patterns in the mutant. Clusters 8 and 9 include the well-studied early meiotic genes, which are de-repressed in the mutant cultured in YPA (but often not in YPD, see Fig. 1) and then persist in SPII. In wild-type yeast, these genes peak at 4 h during sporulation, and decline at the later time points. Cluster 10 shows a category of genes that are induced during early and middle meiosis in wild-type yeast without declining at later phases. These genes are de-repressed in respiring ume6/ume6 cells. Clusters 11 and 12 contain middle meiotic genes and acetate-inducible genes, which fail to be activated to normal levels in ume6 mutant cells that arrest prior to meiotic M-phase. We note that some of these genes may also play a role in temperature stress response because they are induced during the first hour after haploid cells were released from a commonly employed cell cycle synchronization procedure (Orlando et al. 2008).
Fig. 2.
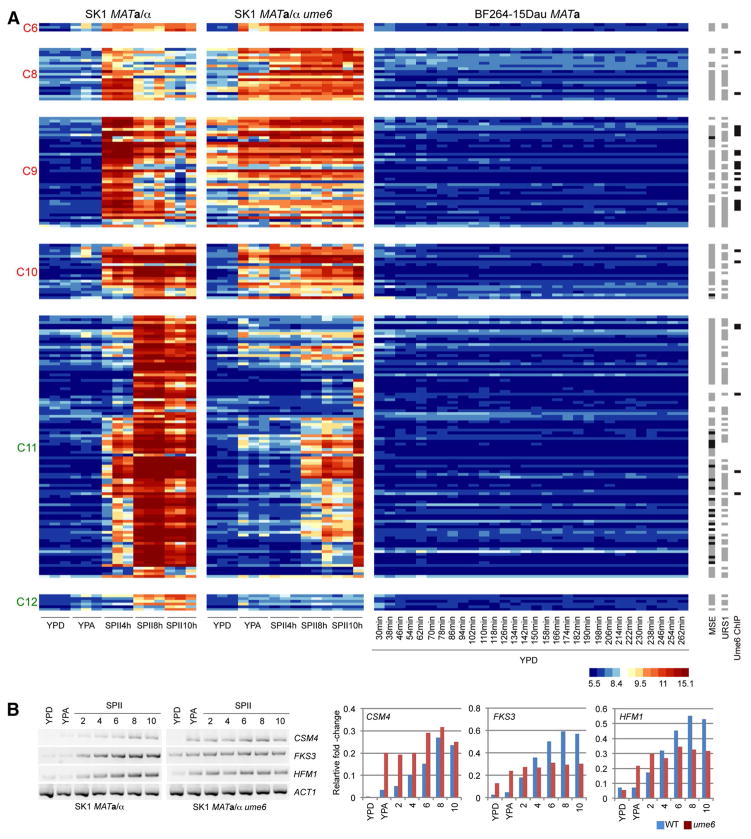
RNA profiles of diploid wild-type and ume6 strains in growth media and sporulation medium versus haploid cells in YPD growth medium. A false-color heatmap is shown for GeneChip expression signals. Every line corresponds to a probeset (transcript) and every column to a sample replicate. Strains are indicated at the top; growth media (YPD, YPA) and time points in sporulation medium (SPII 4, 8 and 10 h) are shown at the bottom. Cluster numbers are given to the left. Predicted Ume6 target motifs (URS1) and in vivo target promoters (Ume6 ChIP) are shown to the right. A color scale for log2-transformed data is given. Red bars mark increased occurrence of Ume6 binding in clusters C6 and C9
Genome-wide in vivo Ume6-DNA binding data are important for identifying direct target genes. These data indicate that Ume6 directly binds the promoters of various loci in all clusters. We find Ume6-promoter interactions to be significantly enriched in Cluster 6 (binomial exact test, p value 2.622 × 10−5), containing genes involved in carbon source metabolism, and Cluster 9 (binomial exact test, p value 1.27 × 10−8), harboring early meiotic genes (Harbison et al. 2004). We sought to further confirm direct Ume6 association with a target gene in our diploid strain background, because high-throughput chromatin precipitation studies are typically carried out in haploid strains. We selected SIP4 (encoding a transcription factor involved in gluconeogenesis), since (1) its mRNA is strongly de-repressed in ume6 mutants cultured in YPD (cluster 8), (2) we find four URS1 motifs in its promoter, and (3) its upstream intergenic region is bound by Ume6 in haploid cells (Online Resource Figure S6A–C) (Williams et al. 2002; Harbison et al. 2004). We find that in fermenting diploid cells, Ume6 binds to the SIP4 promoter in vivo to a level comparable with the known target SPO13 (Online Resource Figure S6D). This indicates that individual binding assays and large-scale assays in haploid and diploid cells yield coherent results for this locus (Fig. 2).
Taken together, our findings permit two conclusions: first, our experiment produced the anticipated pattern of direct targets that increase in ume6/ume6 cells and are associated with upstream URS1 motifs bound by Ume6 in vivo, and indirectly dependent transcripts that fail to accumulate or to decrease because of impaired growth and arrested meiotic development. Second, previously known Ume6-controlled early meiotic genes and metabolic loci represented only a fraction of the complete set of Ume6-dependent genes.
Functional classification of Ume6-dependent genes
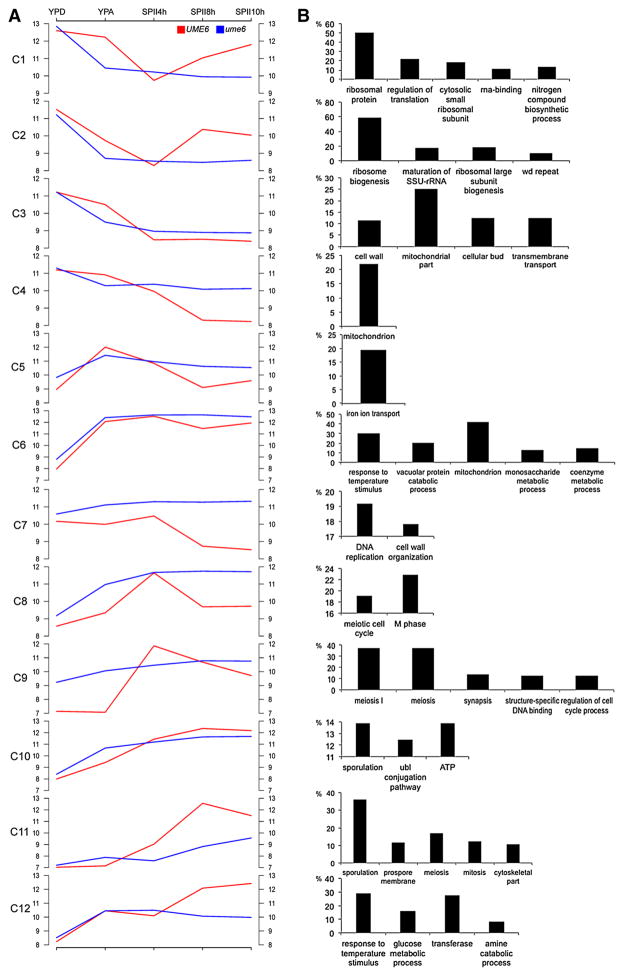
To explore the functional significance of the clusters, we determined which annotation terms from the biological process Gene Ontology were enriched (Huntley et al. 2014). Cells lacking Ume6 fail to efficiently induce genes important for ribosome biogenesis, nitrogen mobilization, mitochondrial functions, and nutrient transport (C1–C5), while loci involved in DNA replication, metabolic processes, cell wall organization, and temperature stress response remain elevated in starving ume6/ume6 cells cultured in SPII medium (C6–C7). Clusters 8 and 9 contain known meiotic functions; while Clusters 10 and 11 are associated with processes such as cell architecture, proteolysis, and spore formation. Cluster 12 indicates that starving mutant cells (after 8–10 h in SPII) fail to induce loci important for metabolic processes during spore formation and maturation (Fig. 3). These results reflect the broad implication of Ume6-dependent gene expression in cell growth, nutritional response, and cell differentiation (see Online Resource File S1 for details).
Fig. 3.
Gene Ontology enrichment in expression clusters. a A graphical display shows the log2-transformed median expression signals in clusters C1–C12 for wild-type cells (UME6) in red and ume6/ume6 mutant cells (ume6) in blue. Samples are indicated at the top. b Bar diagrams show the percentage (y-axis) of enriched GO terms (x-axis) in each cluster (hyper-geometric law, Benjamini correction for multiple testing, p value <0.01; annotations clustered with medium stringency by DAVID (da Huang et al. 2009)
Genes induced in starving ume6/ume6 mutant cells are involved in cell cycle progression, metabolic functions, and stress response
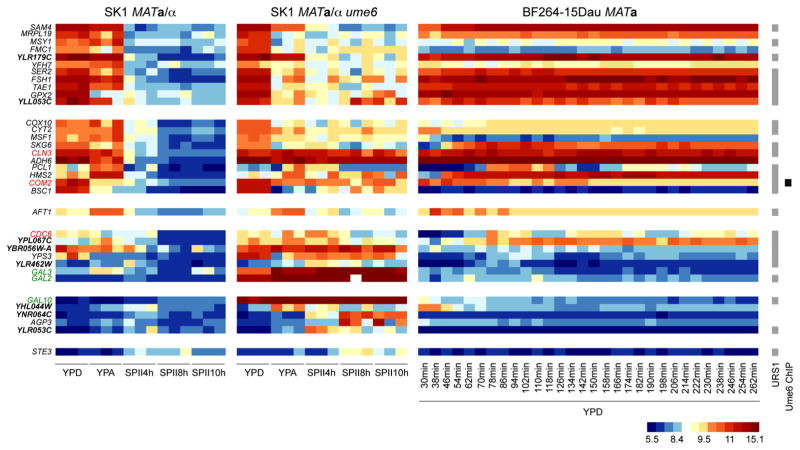
We next asked which genes are not expressed during sporulation in the wild-type strain but exhibit elevated transcript levels in ume6/ume6 mutant cells that arrest prior to entry into meiotic M-phase when cultured in SPII. This approach identified loci falling into six categories (Fig. 4). The first two categories comprise loci that are expressed during growth but not meiotic development in the wild-type, while mutant cells contain measurable transcript levels in SPII medium. These genes are typically important for stress response (FSH1, GPX2, SER2) and mitochondrial functions (MRPL19, FMC1, MSY1, YFH7). Additionally, this category contains two poorly characterized genes (YLR179C, YLL053C), for which our results thus broadly suggest roles in starvation. Interestingly, the second category also includes a negative regulator of the inducer of meiosis IME1 (COM2), a G1 cyclin (CLN3), and two genes important for polarized/pseudohyphal growth (HMS2, PCL1). The third category, which is elevated in YPA, includes only AFT1, an acetate-inducible transcription factor involved in iron homeostasis. The fourth category contains genes typically weakly or not expressed in growing wild-type cells but repressed during sporulation. This class includes a gene essential for DNA replication initiation (CDC6) and three genes of unknown function (YBR056W-A, YLR462W, YPL067C) that may play roles in the cellular starvation response. The genes in the fifth and sixth categories are detected only in ume6 cells and reflect the progressive breakdown of cellular function in sporulation-deficient cells deprived of nutrients, through genes such as AGP3 (amino acid permease), YHL044W (stress response membrane protein), YLR053C (mRNA decay), and YNR064C (detoxification). We note that the ume6/ume6 deletion strain employed in this study and in earlier work (Williams et al. 2002) also lacks the repressor Gal80; we therefore used its targets GAL2, GAL3, and GAL10 as positive controls for up-regulated genes. Functional annotation data we refer to are available at the Saccharomyces Genome Database (www.yeastgenome.org; (Costanzo et al. 2014)). These results reveal molecular events underlying the failure of mutant cells to enter meiotic M-phase after having completed pre-meiotic DNA replication.
Fig. 4.
Gene expression in starving ume6 cells. A heatmap for genes up-regulated in ume6 cells cultured SPII medium is shown like in Fig. 2 for six different classes of patterns. A color scale is given at the bottom. Genes relevant for meiosis and cell cycle progression are given in red. Reference genes are shown in green. Poorly characterized genes are given in bold. Predicted Ume6 target motifs (URS1) are shown to the right. Black bars indicate Ume6 in vivo binding (Ume6 ChIP) (Harbison et al. 2004)
Ume6-dependent genes reflect a comprehensive response of diploid cells to nutritional signals triggering gametogenesis
We next sought to explore genes induced during sporulation but undetectable in asynchronously growing diploid cells (YPD, YPA) or in synchronized haploid fermenting cells (YPD). We expected to identify genes important for Ume6-dependent functions specifically during meiosis and gametogenesis. This step yielded 183 genes falling into two groups. The first group contains 82 genes likely directly repressed by Ume6 [from C6 (3 genes), C8 (19), C9 (40), C10 (20)]. The second group contains 101 genes that appear in most cases to be indirectly dependent on Ume6 [C11 (95) and C12 (6)] as shown in Fig. 5a (for more details on these genes use the filter options in Online Resource File S1). Members of the former group accumulate in YPD or YPA (or both) in the mutant, while those falling into the latter group typically fail to be induced in the mutant because of its meiotic arrest phenotype. Genes directly relevant for the phenotypes shown by ume6/ume6 cells are therefore likely present in the first group (Davey et al. 2012; Shively et al. 2013; Costanzo et al. 2014).
Fig. 5.
Ume6-dependent genes not expressed in YPD. a A heatmap is shown like in Fig. 2. Clusters containing likely direct Ume6 target genes are given in red. Clusters containing indirectly Ume6-dependent genes are shown in green. Strains are indicated at the top. Predicted Ume6 (URS1) and Ndt80 (MSE) sites are shown in gray to the right, known MSEs are represented in black. Black bars indicate in vivo Ume6 binding (Ume6 ChIP) (Harbison et al. 2004). b The output of RT-PCR assays is shown for the target genes as indicated in samples cultured in rich medium (YPD), pre-sporulation medium (YPA), and sporulation medium (SPII) at the time points given. Wild-type (SK1 MATa/α) and mutant strains (SK1 MATa/α ume6) are shown at the bottom. The band intensities were quantified and shown in color-coded histograms where samples (x-axis) are plotted against the relative fold-change (y-axis). Legends indicate the target genes and the color code for the strains
C6 contains a gene important for respiration (MOH1), a Sin3 co-factor (STB2), and a gene that decreases sporulation efficiency when deleted and that promotes invasive growth when over-expressed (LEE1). In all three cases, the mRNA accumulates only in YPA and no Ume6 binding to their upstream region was detected in fermenting haploid cells (Harbison et al. 2004).
C8 notably contains three genes important for meiosis and spore formation (CSM4, PCH2, RMD6), and a gene involved in double-strand break repair (RAD59). The remaining genes are involved in cell division (DOC1, IPL1, MPS2), metabolic functions (AHD7, ATG23, DAL1, GTT2, MCH2, PCD1, SRX1, SUL1, SUL2), and stress response (CIS1). Two poorly characterized loci are implicated in vacuole morphology (YLR173 W) or induce invasive growth upon over-expression (YKL071 W).
C9 includes genes annotated as involved in metabolic functions (DAL4, DAL80, HES1, MET16, PHO92, SRT1), cell division and mating (BNR1, TID3), ribosome biogenesis (RNP1), and DNA repair (BSC4). Furthermore, it contains nearly all loci thought or known to be important for early meiotic functions such as formation of the synaptonemal complex (ECM11, GMC2, HOP1, HOP2, ZIP1, ZIP2), meiotic recombination (DMC1, HFM1, REC8, REC102, REC114, SPO11, MEC1, MEI4, MEI5, MEK1, MND1, MSH4), homolog pairing (NDJ1), meiosis and spore formation (MPC54, SLZ1, SPO1, SPO13, SPO22), meiotic splicing (MER1), and spore wall assembly (FKS3). Given their expression profiles in wild-type cells and the ume6 mutant, three poorly characterized genes (YKR005C, YBR184W, YOL131W) are likely involved in stress response, metabolic functions, or developmental processes. Consistently, cells lacking YKR005C are sensitive to starvation and cells over-expressing the gene undergo invasive growth (Davey et al. 2012; Shively et al. 2013).
C10 comprises genes that accumulate only in ume6 cells cultured in YPA but not YPD that are important for meiosis and spore formation (AMA1, MAM1, CDA1, CDA2, CRR1, ECM8), autophagy (ATG4), translation (HEF3), stress (PAI3, SPG1), and metabolism (DCI1, DSF1, GIP2, PHM6). Five poorly characterized genes are promising candidates for novel Ume6-dependent loci that play roles in cell growth and differentiation (YEL057C, YGR153W, YJL045W, YKL107W, YPL119C-A).
C11 and C12 are clusters of metabolic and sporulation genes that in nearly all cases depend upon Ume6 indirectly, since they are not (or only barely) de-repressed in dividing ume6/ume6 cells and they fail to be induced in arrested mutant cells. These genes typically depend upon the transcriptional activator Ndt80 (which binds the Middle Sporulation Element, MSE). We note that C11 includes three DNA binding transcription factors, including Ndt80, known to be regulated by Ume6 (GAT3, GAT4, NDT80) (Pak and Segall 2002), a gene involved in spore wall assembly (LDS1) and a locus of unknown function (YLR012C), for which promoters are bound by Ume6 in vivo (Harbison et al. 2004). These results extend Ume6’s role in the comprehensive metabolic/stress/meiotic response of a diploid cell to an environmental stimulus triggering meiotic differentiation.
We next sought to validate the GeneChip expression data and selected three genes involved in chromosome segregation in meiosis (CSM4, Cluster 8), the control of spore wall formation via regulation of 1,3-β-glucan synthase (FKS3, Cluster 9), and a DNA helicase family member involved in meiotic recombination (HFM1, Cluster 9). In all cases, we find that RT-PCR assays of wild-type and ume6 mutant samples cultured in growth media (YPD, YPA) and sporulation medium (SPII) reiterate the pattern obtained with GeneChips; ACT1 was used as a loading control (Fig. 5b).
Ume6 target protein network interactions connect proteolysis, meiotic recombination, and nuclear division
We next used protein network data to explore the interactions among Ume6-dependent proteins shown in Fig. 5. The connected component (CC) shown in Fig. 6 links APC/C-dependent protein degradation (DOC1, cluster 8, encoding a co-factor involved in substrate recognition), meiotic recombination (DMC1, cluster 9, encoding a recombinase important for double-strand break repair), and the nuclear division cycle (NDC80, cluster 9, which codes for a component of the kinetochore-associated Ndc80 complex). This CC also highlights possible functions for two poorly characterized proteins (Ypl260w, Ylr456w) in meiotic cell division because they interact with Ndc80.
Network interactions identify hub proteins among Ume6 target genes that are relevant for the ume6/ume6 mutant’s growth and meiotic arrest phenotypes. These findings underline the usefulness of genomic data integration in providing leads for further experimentation exploring mechanisms of how Ume6-dependent epigenetic modifications coordinate different aspects of cell growth and development.
Discussion
We report a genome-wide mRNA-profiling study based on highly reliable Yeast Genome 2.0 GeneChip technology. Our work identified the transcriptome of mitosis and meiosis in the presence and absence of Ume6, which is the DNA binding subunit of chromatin modification enzymes important for normal cell growth and development. We identify direct Ume6 target genes by integrating GeneChip expression profiling data with predicted URS1 motifs and previously published large-scale Ume6-DNA binding data. Furthermore, we link consecutive waves of Ume6-controlled genes via protein network data. The present study therefore represents a rich source of genetic leads for further mechanistic work on elucidating the complex Ume6 gene deletion phenotype.
The usefulness of integrating expression profiling, in vivo protein/DNA binding, and motif predictions
In an earlier RNA profiling experiment using the first generation of Yeast GeneChips (Ye6100), we compared single samples of fermenting (YPD) and respiring (YPA) wild-type control strains to ume6/ume6 mutants in the SK1 and W303 backgrounds; the minimal URS1 motif (5′-GGCGGC-3′ or 5′-CGGCGG-3′) was identified in the promoter regions by iterative scans of the intergenic sequences (Williams et al. 2002). The aim of this experiment was to identify a core set of strain- and carbon source-independent Ume6 target genes. While this previous study indeed identified reference genes repressed by Ume6 and a set of novel targets, it remained incomplete for several reasons. For example, single samples from each strain background rather than true replicates were analyzed, and the Ye6100 GeneChips used were based on very early (hence incomplete and partially erroneous) yeast genome annotation data (Goffeau et al. 1996). In addition, regulatory motif searches did not include information about DNA sequence conservation across related yeasts (Cliften et al. 2003; Kellis et al. 2003) and much less was known about gene function (Costanzo et al. 2014) or protein–protein interactions (Orchard et al. 2014). Finally, no data were available for synchronized mitotically diving cells (Orlando et al. 2008) and genome-wide in vivo Ume6/DNA binding patterns (Harbison et al. 2004).
The present study was designed to rectify these issues and thus identify the largest possible set of transcripts that statistically significantly depends on Ume6 (hence, its interactors such as Rpd3 and Isw2), in the strain background that is most relevant for analyses of growth and meiotic development (SK1). To distinguish direct from indirect targets, we used URS1 motif predictions and in vivo Ume6 DNA binding data. While our approach turned out to be efficient, some issues remain—such as incoherent data. For example, promoters were identified that are bound in vivo by Ume6, possess a URS1 motif, but the transcript expression level does not change in the mutant strain. We recently found an explanation for this phenomenon: many yeast loci encode multiple isoforms, only one of which is regulated by Ume6 (Lardenois et al. 2015; Liu et al. 2015; Stuparevic et al. 2015). Such cases cannot be identified with Yeast Genome 2.0 GeneChips because they cover only a small sequence within the target open reading frame’s 3′-region. Other confounding factors include Ume6 binding sites other than the canonical URS1 motif (Sweet et al. 1997) or the ability of Ume6 to recruit factors onto promoters independently of its target motifs within three-dimensional chromatin structures (Yadon et al. 2010).
In general, distinct transcript levels obtained with microarrays are attributed to transcriptional effects (especially in the case of DNA binding transcription factors). However, mechanisms unrelated to mRNA synthesis can also influence signal intensities, such as changes in transcript half-life or elevated chromosomal instability resulting in multiple chromosomes in a mutant strain. In this context, we note that haploid ume6 cells in W303 and S288C backgrounds are reported to contain two copies of chromosomes 9 and 16 (among other alterations) (Fazzio et al. 2001). We do not know if similar effects occur in diploid SK1 ume6/ume6 cells but we cannot exclude the possibility. Therefore, RNA profiling data indicating 2-fold changes of transcript levels for genes located on chromosomes 9 and 16 should be interpreted with the caveat of polyploidy in mind. We note, in any case, that typical bona fide Ume6 target genes for which Ume6 binds a URS1 motif in vivo show higher than twofold changes and are therefore unlikely to be affected by the issue.
Why do cells lacking Ume6 fail to undergo meiosis?
It seems paradoxical that a transcriptional repressor whose destruction is required for meiotic progression should be essential for the process. A trivial explanation is that mutant cells have a growth phenotype that perturbs mitosis such that efficient entry into meiosis is not possible. Ume6 is indeed important for normal cell divisions and in the Σ1278b strain background it is even essential for growth (Strich et al. 1994, 2011; Suzuki et al. 2003). However, in our SK1 and JHY222 backgrounds and earlier work using W303, we did not observe a mitotic phenotype strong enough to explain the arrest of ume6 mutant cells after pre-meiotic DNA replication (Williams et al. 2002). As in the case of the 3′-5′ exoribonuclease Rrp6, which is also degraded during meiotic M-phase and required for normal sporulation (Lardenois et al. 2015), it is likely the case that altered levels of proteins important for the transition between mitotic growth and meiotic development are at least partially responsible for the phenotype. For example, we observe that COM2, a negative regulator of IME1 (Inducer of Meiosis 1), remains transcriptionally active in ume6/ume6 cells cultured in sporulation medium (Kahana-Edwin et al. 2013). We also find that the DNA replication activator CDC6 and the G1 cyclin CLN3 (which promotes entry into the mitotic cell division cycle) fail to be transcriptionally down-regulated prior to entry into meiotic M-phase (Ofir et al. 2004; Shi and Tu 2013). It is, however, difficult to discern if this effect is the cause or the consequence of the ume6/ume6 mutant’s meiotic cell cycle arrest.
Fermenting and/or respiring cells that lack Ume6 derepress a number of genes that can interfere with normal mitotic growth when over-expressed, such as ADY3, MAM1, NDJ1, RME1, RED1, SPO13, and SPS22 (McCarroll and Esposito 1994; Sopko et al. 2006; Varela et al. 2010). We do not know if all meiotic mRNAs are efficiently translated during mitotic growth like it is the case for SPO13; however, it is plausible that at least some of them are, and that the combined effects of several inhibitory proteins brings the mitosis–meiosis transition to a halt before mutant cells can enter meiotic M-phase. Another explanation might be the premature accumulation of developmental stage-specific proteins involved in meiotic recombination (e.g., REC114, SPO11, DMC1), formation of the synaptonemal complex (including HOP1, HOP2, ZIP1, ZIP2), and segregation of meiotic chromosomes (CSM4), which may trigger a checkpoint. While this remains speculative, we note that NDC80, encoding a protein involved in chromosome segregation and spindle checkpoint activity, and PCH2, encoding a component of the pachytene checkpoint, are induced in ume6/ume6 cells cultured in growth media (see Saccharomyces Genome Database for gene annotation references, (Costanzo et al. 2014), GermOnline for expression data (Lardenois et al. 2010), Online Resource File S1).
Ume6 is tightly regulated at the level of protein stability by the APC/C when diploid cells switch from fermentation to respiration and then sporulation (Mallory et al. 2007, 2012; Law et al. 2014). Interestingly, we find that DOC1, which is important for the APC/C’s ability to recognize its substrates and to ubiquitinate them, is induced in cells lacking Ume6 (Online Resource File S1) (Carroll and Morgan 2002; Passmore et al. 2003). This points to a possible negative feedback loop mechanism between Ume6 and the APC/C, whereby Ume6 partially inhibits the APC/C by keeping DOC1 expression low. As Ume6 levels diminish during the onset of meiosis, DOC1 expression increases, which might accelerate Ume6 degradation.
Conclusion and outlook
Given that the budding yeast transcriptome comprises mRNAs, developmental stage-specific mRNA isoforms with extended 5′- and 3′-UTRs and long non-coding RNAs (lncRNAs), it seems reasonable to assume that Ume6 is not only important for the former two transcript categories, but also for RNAs with little or no coding potential (Cho et al. 1998; Chu et al. 1998; Primig et al. 2000; Wyers et al. 2005; Nagalakshmi et al. 2008; Xu et al. 2009; Lardenois et al. 2011, 2014; Kim Guisbert et al. 2012; Waern and Snyder 2013). Indeed, we have observed by RT-PCR assays that ume6/ume6 and rpd3/rpd3 mutant cells cultured in YPD and YPA accumulate a number of lncRNAs, such as meiotic unannotated transcripts (MUTs), cryptic unstable transcripts (CUTs), and stable unannotated transcripts (SUTs) to higher levels than the wild-type control strain (Y. Liu and B. Xie, unpublished). Genome-wide DNA strand-specific RNA profiling using RNA-Sequencing will answer the question to what extent lncRNAs are controlled by Ume6-dependent epigenetic mechanisms, and if their abnormal accumulation in mitotic cells contributes to the complex growth and developmental phenotype of ume6/ume6 mutant cells.
Acknowledgments
Michael Primig extends his special thanks to Rochelle Easton Esposito for her support, guidance, and mentorship during several years he spent with her as a postdoctoral researcher at the University of Chicago and as an assistant professor at the Biozentrum in Basel. We thank Olivier Collin and Olivier Sallou for GermOnline systems administration and Aaron Mitchell for the SK1 MATa/α ume6/ume6 mutant strain. This work was supported by a Young Investigator fellowship from the Institut National de Santé et de Recherche Médicale (Inserm) awarded to A. Lardenois and an Inserm Avenir grant (R07216NS) awarded to M. Primig.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00438-015-1051-5) contains supplementary material, which is available to authorized users.
Conflict of interest: The authors disclose no conflicts of interest.
Ethical standards: The research does not involve human participants or animals.
References
- Anderson SF, Steber CM, et al. UME6, a negative regulator of meiosis in Saccharomyces cerevisiae, contains a C-terminal Zn2Cys6 binuclear cluster that binds the URS1 DNA sequence in a zinc-dependent manner. Protein Sci. 1995;4(9):1832–1843. doi: 10.1002/pro.5560040918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Uppuluri P, et al. Expression of UME6, a key regulator of Candida albicans hyphal development, enhances biofilm formation via Hgc1- and Sun41-dependent mechanisms. Eukaryot Cell. 2013;12(2):224–232. doi: 10.1128/EC.00163-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew CR, Suzuki T, et al. Ume6 transcription factor is part of a signaling cascade that regulates autophagy. Proc Natl Acad Sci USA. 2012;109(28):11206–11210. doi: 10.1073/pnas.1200313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baryshnikova A, Costanzo M, et al. Synthetic genetic array (SGA) analysis in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Methods Enzymol. 2010;470:145–179. doi: 10.1016/S0076-6879(10)70007-0. [DOI] [PubMed] [Google Scholar]
- Carlisle PL, Kadosh D. Candida albicans Ume6, a filament-specific transcriptional regulator, directs hyphal growth via a pathway involving Hgc1 cyclin-related protein. Eukaryot Cell. 2010;9(9):1320–1328. doi: 10.1128/EC.00046-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle PL, Banerjee M, et al. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci USA. 2009;106(2):599–604. doi: 10.1073/pnas.0804061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CW, Morgan DO. The Doc1 subunit is a processivity factor for the anaphase-promoting complex. Nat Cell Biol. 2002;4(11):880–887. doi: 10.1038/ncb871. [DOI] [PubMed] [Google Scholar]
- Chalmel F, Rolland AD, et al. The conserved transcriptome in human and rodent male gametogenesis. Proc Natl Acad Sci USA. 2007;104(20):8346–8351. doi: 10.1073/pnas.0701883104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers DS, Mundodi V, et al. A 5′ UTR-mediated translational efficiency mechanism inhibits the Candida albicans morphological transition. Mol Microbiol. 2014;92(3):570–585. doi: 10.1111/mmi.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RJ, Campbell MJ, et al. A genome-wide transcriptional analysis of the mitotic cell cycle. Mol Cell. 1998;2(1):65–73. doi: 10.1016/s1097-2765(00)80114-8. [DOI] [PubMed] [Google Scholar]
- Chu S, DeRisi J, et al. The transcriptional program of sporulation in budding yeast. Science. 1998;282(5389):699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- Cliften P, Sudarsanam P, et al. Finding functional features in Saccharomyces genomes by phylogenetic footprinting. Science. 2003;301(5629):71–76. doi: 10.1126/science.1084337. [DOI] [PubMed] [Google Scholar]
- Costanzo MC, Engel SR, et al. Saccharomyces genome database provides new regulation data. Nucleic Acids Res. 2014;42(Database issue):D717–D725. doi: 10.1093/nar/gkt1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Huang W, Sherman BT, et al. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Davey HM, Cross EJ, et al. Genome-wide analysis of longevity in nutrient-deprived Saccharomyces cerevisiae reveals importance of recycling in maintaining cell viability. Environ Microbiol. 2012;14(5):1249–1260. doi: 10.1111/j.1462-2920.2012.02705.x. [DOI] [PubMed] [Google Scholar]
- de Lichtenberg U, Jensen LJ, et al. Dynamic complex formation during the yeast cell cycle. Science. 2005;307(5710):724–727. doi: 10.1126/science.1105103. [DOI] [PubMed] [Google Scholar]
- Fazzio TG, Kooperberg C, et al. Widespread collaboration of Isw2 and Sin3-Rpd3 chromatin remodeling complexes in transcriptional repression. Mol Cell Biol. 2001;21(19):6450–6460. doi: 10.1128/MCB.21.19.6450-6460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene Ontology C. Gene ontology consortium: going forward. Nucleic Acids Res. 2015;43(Database issue):D1049–D1056. doi: 10.1093/nar/gku1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau A, Barrell BG, et al. Life with 6000 genes. Science. 1996;274(5287):546, 563–567. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- Goldmark JP, Fazzio TG, et al. The Isw2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell. 2000;103(3):423–433. doi: 10.1016/s0092-8674(00)00134-3. [DOI] [PubMed] [Google Scholar]
- Harbison CT, Gordon DB, et al. Transcriptional regulatory code of a eukaryotic genome. Nature. 2004;431(7004):99–104. doi: 10.1038/nature02800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenmeyer ME, Fung E, et al. The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science. 2008;320(5874):362–365. doi: 10.1126/science.1150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley RP, Sawford T, et al. The GOA database: gene ontology annotation updates for 2015. Nucleic Acids Res. 2014 doi: 10.1093/nar/gku1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isserlin R, El-Badrawi RA, et al. The biomolecular interaction network database in PSI-MI 2.5. Database (Oxford) 2011:baq037. doi: 10.1093/database/baq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh D, Struhl K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell. 1997;89(3):365–371. doi: 10.1016/s0092-8674(00)80217-2. [DOI] [PubMed] [Google Scholar]
- Kahana-Edwin S, Stark M, et al. Multiple MAPK cascades regulate the transcription of IME1, the master transcriptional activator of meiosis in Saccharomyces cerevisiae. PLoS One. 2013;8(11):e78920. doi: 10.1371/journal.pone.0078920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassir Y, Adir N, et al. Transcriptional regulation of meiosis in budding yeast. Int Rev Cytol. 2003;224:111–171. doi: 10.1016/s0074-7696(05)24004-4. [DOI] [PubMed] [Google Scholar]
- Kellis M, Patterson N, et al. Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature. 2003;423(6937):241–254. doi: 10.1038/nature01644. [DOI] [PubMed] [Google Scholar]
- Kim Guisbert KS, Zhang Y, et al. Meiosis-induced alterations in transcript architecture and noncoding RNA expression in S. cerevisiae. RNA. 2012;18(6):1142–1153. doi: 10.1261/rna.030510.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzer S, Schuller HJ. Transcriptional control of the yeast acetyl-CoA synthetase gene, ACS1, by the positive regulators CAT8 and ADR1 and the pleiotropic repressor UME6. Mol Microbiol. 1997;26(4):631–641. doi: 10.1046/j.1365-2958.1997.5611937.x. [DOI] [PubMed] [Google Scholar]
- Kurdistani SK, Robyr D, et al. Genome-wide binding map of the histone deacetylase Rpd3 in yeast. Nat Genet. 2002;31(3):248–254. doi: 10.1038/ng907. [DOI] [PubMed] [Google Scholar]
- Lardenois A, Gattiker A, et al. GermOnline 4.0 is a genomics gateway for germline development, meiosis and the mitotic cell cycle. Database: the journal of biological databases and curation 2010. 2010:baq030. doi: 10.1093/database/baq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardenois A, Liu Y, et al. Execution of the meiotic noncoding RNA expression program and the onset of gametogenesis in yeast require the conserved exosome subunit Rrp6. Proc Natl Acad Sci USA. 2011;108(3):1058–1063. doi: 10.1073/pnas.1016459108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardenois A, Stuparevic I, et al. The conserved histone deacetylase Rpd3 and its DNA binding subunit Ume6 control dynamic transcript architecture during mitotic growth and meiotic development. Nucleic Acids Res. 2015;43(1):115–128. doi: 10.1093/nar/gku1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MJ, Mallory MJ, et al. Acetylation of the transcriptional repressor Ume6p allows efficient promoter release and timely induction of the meiotic transient transcription program in yeast. Mol Cell Biol. 2014;34(4):631–642. doi: 10.1128/MCB.00256-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Stuparevic I, et al. The conserved histone deacetylase Rpd3 and the DNA binding regulator Ume6 repress BOI1’s meiotic transcript isoform during vegetative growth in Saccharomyces cerevisiae. Mol Microbiol. 2015 doi: 10.1111/mmi.12976. [DOI] [PubMed] [Google Scholar]
- Mallory MJ, Cooper KF, et al. Meiosis-specific destruction of the Ume6p repressor by the Cdc20-directed APC/C. Mol Cell. 2007;27(6):951–961. doi: 10.1016/j.molcel.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory MJ, Law MJ, et al. Gcn5p-dependent acetylation induces degradation of the meiotic transcriptional repressor Ume6p. Mol Biol Cell. 2012;23(9):1609–1617. doi: 10.1091/mbc.E11-06-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll RM, Esposito RE. SPO13 negatively regulates the progression of mitotic and meiotic nuclear division in Saccharomyces cerevisiae. Genetics. 1994;138(1):47–60. doi: 10.1093/genetics/138.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F, Vierendeels F, et al. In Saccharomyces cerevisiae, expression of arginine catabolic genes CAR1 and CAR2 in response to exogenous nitrogen availability is mediated by the Ume6 (CargRI)-Sin3 (CargRII)-Rpd3 (CargRIII) complex. J Bacteriol. 2000;182(11):3158–3164. doi: 10.1128/jb.182.11.3158-3164.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaillat L, Mayer A. Identification of genes affecting vacuole membrane fragmentation in Saccharomyces cerevisiae. PLoS One. 2013;8(2):e54160. doi: 10.1371/journal.pone.0054160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AP. Control of meiotic gene expression in Saccharomyces cerevisiae. Microbiol Rev. 1994;58(1):56–70. doi: 10.1128/mr.58.1.56-70.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalakshmi U, Wang Z, et al. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320(5881):1344–1349. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngounou Wetie AG, Sokolowska I, et al. Protein-protein interactions: switch from classical methods to proteomics and bioinformatics-based approaches. Cellular and molecular life sciences : CMLS. 2014;71(2):205–228. doi: 10.1007/s00018-013-1333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nookaew I, Papini M, et al. A comprehensive comparison of RNA-Seq-based transcriptome analysis from reads to differential gene expression and cross-comparison with microarrays: a case study in Saccharomyces cerevisiae. Nucleic Acids Res. 2012;40(20):10084–10097. doi: 10.1093/nar/gks804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor L, Caplice N, et al. Differential filamentation of Candida albicans and Candida dubliniensis Is governed by nutrient regulation of UME6 expression. Eukaryot Cell. 2010;9(9):1383–1397. doi: 10.1128/EC.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir Y, Sagee S, et al. The role and regulation of the preRC component Cdc6 in the initiation of premeiotic DNA replication. Mol Biol Cell. 2004;15(5):2230–2242. doi: 10.1091/mbc.E03-08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard S, Ammari M, et al. The MIntAct project—IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res. 2014;42(Database issue):D358–D363. doi: 10.1093/nar/gkt1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein Y, Linhart C, et al. Assessment of algorithms for inferring positional weight matrix motifs of transcription factor binding sites using protein binding microarray data. PLoS One. 2012;7(9):e46145. doi: 10.1371/journal.pone.0046145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando DA, Lin CY, et al. Global control of cell-cycle transcription by coupled CDK and network oscillators. Nature. 2008;453(7197):944–947. doi: 10.1038/nature06955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak J, Segall J. Regulation of the premiddle and middle phases of expression of the NDT80 gene during sporulation of Saccharomyces cerevisiae. Mol Cell Biol. 2002;22(18):6417–6429. doi: 10.1128/MCB.22.18.6417-6429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HD, Luche RM, et al. The yeast UME6 gene product is required for transcriptional repression mediated by the CAR1 URS1 repressor binding site. Nucleic Acids Res. 1992;20(8):1909–1915. doi: 10.1093/nar/20.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore LA, McCormack EA, et al. Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. EMBO J. 2003;22(4):786–796. doi: 10.1093/emboj/cdg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primig M, Williams RM, et al. The core meiotic transcriptome in budding yeasts. Nat Genet. 2000;26(4):415–423. doi: 10.1038/82539. [DOI] [PubMed] [Google Scholar]
- Prinz S, Avila-Campillo I, et al. Control of yeast filamentous-form growth by modules in an integrated molecular network. Genome Res. 2004;14(3):380–390. doi: 10.1101/gr.2020604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundlett SE, Carmen AA, et al. Transcriptional repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature. 1998;392(6678):831–835. doi: 10.1038/33952. [DOI] [PubMed] [Google Scholar]
- Rustici G, Kolesnikov N, et al. ArrayExpress update–trends in database growth and links to data analysis tools. Nucleic Acids Res. 2013;41(Database issue):D987–D990. doi: 10.1093/nar/gks1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R, Smoot ME, et al. A travel guide to Cytoscape plugins. Nat Methods. 2012;9(11):1069–1076. doi: 10.1038/nmeth.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Tu BP. Acetyl-CoA induces transcription of the key G1 cyclin CLN3 to promote entry into the cell division cycle in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2013;110(18):7318–7323. doi: 10.1073/pnas.1302490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Eckwahl MJ, et al. Genetic networks inducing invasive growth in Saccharomyces cerevisiae identified through systematic genome-wide overexpression. Genetics. 2013;193(4):1297–1310. doi: 10.1534/genetics.112.147876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopko R, Huang D, et al. Mapping pathways and phenotypes by systematic gene overexpression. Mol Cell. 2006;21(3):319–330. doi: 10.1016/j.molcel.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Spivak AT, Stormo GD. ScerTF: a comprehensive database of benchmarked position weight matrices for Saccharomyces species. Nucleic Acids Res. 2012;40(Database issue):D162–D168. doi: 10.1093/nar/gkr1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strich R, Slater MR, et al. Identification of negative regulatory genes that govern the expression of early meiotic genes in yeast. Proc Natl Acad Sci USA. 1989;86(24):10018–10022. doi: 10.1073/pnas.86.24.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strich R, Surosky RT, et al. UME6 is a key regulator of nitrogen repression and meiotic development. Genes Dev. 1994;8(7):796–810. doi: 10.1101/gad.8.7.796. [DOI] [PubMed] [Google Scholar]
- Strich R, Khakhina S, et al. Ume6p is required for germination and early colony development of yeast ascospores. FEMS Yeast Res. 2011;11(1):104–113. doi: 10.1111/j.1567-1364.2010.00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuparevic I, Becker E, et al. The histone deacetylase Rpd3/Sin3/Ume6 complex represses an acetate-inducible isoform of VTH2 in fermenting budding yeast cells. FEBS Lett. 2015 doi: 10.1016/j.febslet.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Suzuki C, Hori Y, et al. Screening and characterization of transposon-insertion mutants in a pseudohyphal strain of Saccharomyces cerevisiae. Yeast. 2003;20(5):407–415. doi: 10.1002/yea.970. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Jang YK, et al. Role of UME6 in transcriptional regulation of a DNA repair gene in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17(11):6223–6235. doi: 10.1128/mcb.17.11.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333(1–2):169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- Varela E, Schlecht U, et al. Mitotic expression of Spo13 alters M-phase progression and nucleolar localization of Cdc14 in budding yeast. Genetics. 2010;185(3):841–854. doi: 10.1534/genetics.109.113746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waern K, Snyder M. Extensive transcript diversity and novel upstream open reading frame regulation in yeast. G3 (Bethesda) 2013;3(2):343–352. doi: 10.1534/g3.112.003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RM, Primig M, et al. The Ume6 regulon coordinates metabolic and meiotic gene expression in yeast. Proc Natl Acad Sci USA. 2002;99(21):13431–13436. doi: 10.1073/pnas.202495299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender E. The TRANSFAC project as an example of framework technology that supports the analysis of genomic regulation. Brief Bioinform. 2008;9(4):326–332. doi: 10.1093/bib/bbn016. [DOI] [PubMed] [Google Scholar]
- Wyers F, Rougemaille M, et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell. 2005;121(5):725–737. doi: 10.1016/j.cell.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wei W, et al. Bidirectional promoters generate pervasive transcription in yeast. Nature. 2009;457(7232):1033–1037. doi: 10.1038/nature07728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadon AN, Van de Mark D, et al. Chromatin remodeling around nucleosome-free regions leads to repression of noncoding RNA transcription. Mol Cell Biol. 2010;30(21):5110–5122. doi: 10.1128/MCB.00602-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K, Tanaka T, et al. Comprehensive phenotypic analysis of single-gene deletion and overexpression strains of Saccharomyces cerevisiae. Yeast. 2011;28(5):349–361. doi: 10.1002/yea.1843. [DOI] [PubMed] [Google Scholar]
- Zeidler U, Lettner T, et al. UME6 is a crucial downstream target of other transcriptional regulators of true hyphal development in Candida albicans. FEMS Yeast Res. 2009;9(1):126–142. doi: 10.1111/j.1567-1364.2008.00459.x. [DOI] [PubMed] [Google Scholar]