Fig. 2.
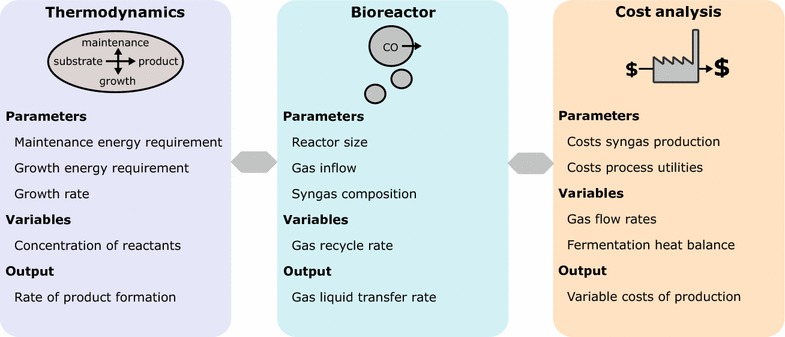
Study approach. The presented model to estimate the variable costs of acetone production from CO with M. thermoacetica can be broken down into 3 parts. Thermodynamics: assuming an energy requirement of 62 kJ/C-mol/h for maintenance, and 1000 kJ/C-mol for growth, and a specific growth rate of 0.10 h−1, the process reaction was established. The process reaction, which describes the rate of conversion of CO, H2O, and the nitrogen source to CO2, cell mass, and acetone, is depending on the concentration of the reactants. The concentration of the gases and acetone in liquid was determined by taking gas–liquid mass transfer limitations into account. Bioreactor: the reactor dimensions (30 m height, 6 m diameter), the gas inflow rate R in, and the composition of the syngas were fixed. The gas transfer rate into the liquid under the chosen process conditions was determined depending on the ratio of fresh and recycled gas. The gas transfer rate determines the amount of substrate that is available to the cell mass and was used as input in the process reaction. For the thermodynamic calculations and calculations on gas–liquid mass transfer, the process temperature of 60 °C was taken into account. Cost analysis: the production rate of the whole plant was set to 30 kt/year and determined eventually the sizing of the plant as well as the variable costs of production
