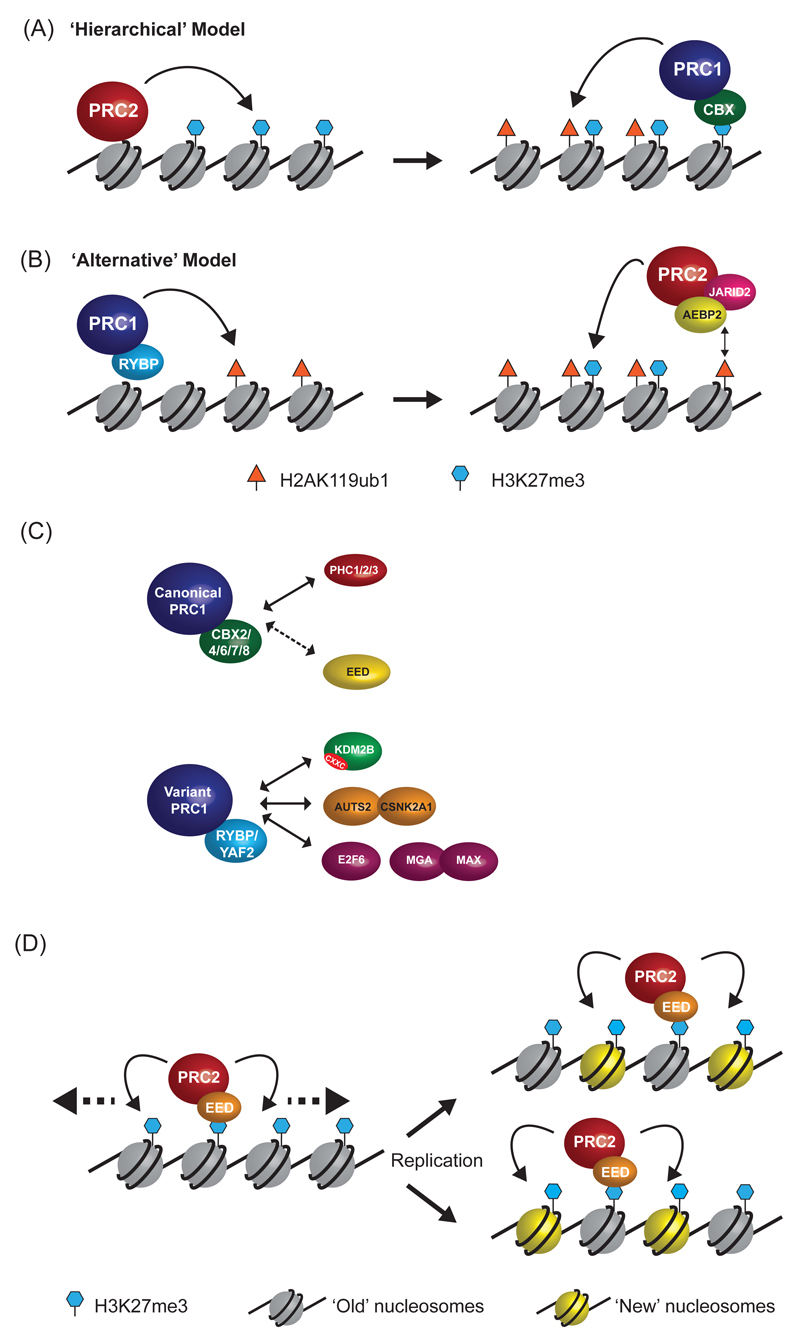
Figure 2. Beyond simple recruitment to more complex interactions.
a | In the ‘hierarchical’ model of polycomb complex function, PRC2 first binds to chromatin and places H3K27me3. It is then proposed that H3K27me3 is recognized by chromobox (CBX) proteins, which are a subunit of canonical PRC1 complexes, thereby allowing PRC1 to bind and mono-ubiquitylate H2AK119.
b | In the ‘Alternative’ model for polycomb recruitment, the initial event is the binding of variant PRC1 complexes (which contain RYBP instead of CBX) to chromatin. PRC1 then catalyses the mono-ubiquitylation of H2AK119, independently of PRC2 activity and H3K27me3. The H2AK119ub1 modification then promotes the recruitment of PRC2, possibly via direct recognition of H2AK119ub1 by the AEBP2-JARID2-PRC2 complex, and placement of the H3K27me3 mark.
c | PRC1 complexes functionally segregate into ‘canonical’ complexes that contain chromobox (CBX2/4/6/7/8) and polyhomeotic (PHC1/2/3) proteins, and a series of ‘variant’ complexes that contain RYBP (or the closely related YAF2 protein) and interact with proteins that are unique to individual variant PRC1 complexes (note that only selected proteins are shown here). Under some conditions the PRC2 subunit EED may interact with a CBX-containing canonical PRC1 complex (dashed arrow).
d | A model for the propagation of polycomb domains.Within the core PRC2 complex, the EED subunit is able to recognise H3K27me3 via its WD40 repeat domain. This interaction potentially recruits PRC2 to sites of pre-existing H3K27me3, as well as stimulating the enzymatic activity of PRC2. The EED-H3K27me3 interaction may facilitate spreading of H3K27me3 domains (left panel, dashed arrows) or copying of H3K27me3 onto newly incorporated histones (right panel, blue nucleosomes) during DNA replication, thereby stably propagating H3K27me3 domains in actively dividing cells.